Paramyxovirus entry
- PMID: 23884588
- PMCID: PMC8782154
- DOI: 10.1007/978-1-4614-7651-1_6
Paramyxovirus entry
Abstract
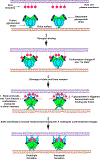
The family Paramyxoviridae consists of a group of large, enveloped, negative-sense, single-stranded RNA viruses and contains many important human and animal pathogens. Molecular and biochemical characterization over the past decade has revealed an extraordinary breadth of biological diversity among this family of viruses. Like all enveloped viruses, paramyxoviruses must fuse their membrane with that of a receptive host cell as a prerequisite for viral entry and infection. Unlike most other enveloped viruses, the vast majority of paramyxoviruses contain two distinct membrane-anchored glycoproteins to mediate the attachment, membrane fusion and particle entry stages of host cell infection. The attachment glycoprotein is required for virion attachment and the fusion glycoprotein is directly involved in facilitating the merger of the viral and host cell membranes. Here we detail important functional, biochemical and structural features of the attachment and fusion glycoproteins from a variety of family members. Specifically, the three different classes of attachment glycoproteins are discussed, including receptor binding preference, their overall structure and fusion promotion activities. Recently solved atomic structures of certain attachment glycoproteins are summarized, and how they relate to both receptor binding and fusion mechanisms are described. For the fusion glycoprotein, specific structural domains and their proposed role in mediating membrane merger are illustrated, highlighting the important features of protease cleavage and associated tropism and virulence. The crystal structure solutions of both an uncleaved and a cleavage-activated metastable F are also described with emphasis on how small conformational changes can provide the necessary energy to mediate membrane fusion. Finally, the different proposed fusion models are reviewed, featuring recent experimental findings that speculate how the attachment and fusion glycoproteins work in concert to mediate virus entry.
Figures

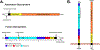
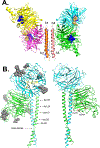

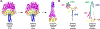

References
-
- Lamb RA, and Parks GD. Paramyxoviridae: The viruses and their replication. In Knipe DM and Howley PM ed., Fields Virology, 5 ed, Philadelphia. Lippincott Williams & Wilkins, 2007:1449–1496.
-
- Alexander DJ. Newcastle disease and other avian paramyxoviruses. Rev Sci Tech 2000; 19(2):443–462. - PubMed
-
- Tambi EN, Maina OW, Mukhebi AW, et al. Economic impact assessment of rinderpest control in Africa. Rev Sci Tech 1999; 18(2):458–477. - PubMed
-
- Eaton BT, Mackenzie JS, and Wang L-F. Henipaviruses. In Knipe DM and Howley PM ed., Fields Virology, 5 ed, Philadelphia. Lippincott Williams & Wilkins, 2007:1587–1600.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

