Iodine quantification with dual-energy CT: phantom study and preliminary experience with VX2 residual tumour in rabbits after radiofrequency ablation
- PMID: 23884759
- PMCID: PMC3755393
- DOI: 10.1259/bjr.20130143
Iodine quantification with dual-energy CT: phantom study and preliminary experience with VX2 residual tumour in rabbits after radiofrequency ablation
Abstract
Objective: The purpose of our study was to validate iodine quantification in a phantom study with dual-source dual-energy CT (DECT) and to apply this technique to differentiate benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after radiofrequency ablation (RFA).
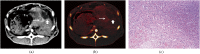
Methods: We applied iodine quantification with DECT in a phantom and in VX2 carcinoma in rabbits after incomplete RFA to differentiate benign periablational reactive tissue from residual tumour and evaluated its efficacy in demonstrating response to therapeutic RFA. A series of tubes containing solutions of varying iodine concentration were scanned with DECT. The iodine concentration was calculated and compared with known true iodine concentration. Triple-phase contrast-enhanced DECT data on 24 rabbits with VX2 carcinoma were then assessed at Day 3 (n=6), 1 week (n=6), 2 weeks (n=6) and 3 weeks (n=6) after incomplete RFA independently by 2 readers. Dual-energy postprocessing was used to produce iodine-only images. Regions of interest were positioned on the iodine image over the lesion and, as a reference, over the aorta, to record iodine concentration in the lesion and in the aorta. The pathological specimens were sectioned in the same plane as DECT imaging, and the lesion iodine concentration and lesion-to-aorta iodine ratio of residual tumour and benign periablational reactive tissue were assessed.
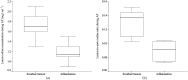
Results: There was excellent correlation between calculated and true iodine concentration (r=0.999, p<0.0001) in the phantom study. The lesion iodine concentration and lesion-to-aorta iodine ratio in residual tumour were significantly higher than in benign periablational reactive tissue in the 2-week group during the arterial phase (AP) (p<0.01) and in the 3-week group during both the AP (p<0.05) and the portal venous phase (p<0.05). There was no significant difference between them with respect to the lesion iodine concentration or lesion-to-aorta iodine ratio in the 3-day and 1-week groups.
Conclusion: Iodine quantification with DECT is accurate in a phantom study and can be used to differentiate benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA.
Advances in knowledge: Iodine quantification with DECT may help in differentiating benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA.
Figures


Similar articles
-
VX2 carcinoma in rabbits after radiofrequency ablation: comparison of MR contrast agents for help in differentiating benign periablational enhancement from residual tumor.Radiology. 2005 Feb;234(2):423-30. doi: 10.1148/radiol.2342031456. Epub 2004 Dec 10. Radiology. 2005. PMID: 15591437
-
Iodine quantification with dual-energy CT: phantom study and preliminary experience with renal masses.AJR Am J Roentgenol. 2011 Jun;196(6):W693-700. doi: 10.2214/AJR.10.5541. AJR Am J Roentgenol. 2011. PMID: 21606256
-
A Method for Reducing Variability Across Dual-Energy CT Manufacturers in Quantification of Low Iodine Content Levels.AJR Am J Roentgenol. 2022 Apr;218(4):746-755. doi: 10.2214/AJR.21.26714. Epub 2021 Oct 20. AJR Am J Roentgenol. 2022. PMID: 34668387
-
Stress Test of Contrast-Enhanced US with Phenylephrine in a Rabbit VX2 Liver Tumor Model: Differentiating Benign Periablational Enhancement from Residual Tumor after Radiofrequency Ablation.J Vasc Interv Radiol. 2016 Jul;27(7):1077-1085.e2. doi: 10.1016/j.jvir.2016.02.012. Epub 2016 Apr 23. J Vasc Interv Radiol. 2016. PMID: 27117950
-
Quantification of liver fat in the presence of iron and iodine: an ex-vivo dual-energy CT study.Invest Radiol. 2011 Jun;46(6):351-8. doi: 10.1097/RLI.0b013e31820e1486. Invest Radiol. 2011. PMID: 21263329
Cited by
-
Noise suppression for dual-energy CT via penalized weighted least-square optimization with similarity-based regularization.Med Phys. 2016 May;43(5):2676. doi: 10.1118/1.4947485. Med Phys. 2016. PMID: 27147376 Free PMC article.
-
A Review of Imaging Methods to Assess Ultrasound-Mediated Ablation.BME Front. 2022;2022:9758652. doi: 10.34133/2022/9758652. Epub 2022 May 2. BME Front. 2022. PMID: 35957844 Free PMC article.
-
Dual-energy CT: a phantom comparison of different platforms for abdominal imaging.Eur Radiol. 2018 Jul;28(7):2745-2755. doi: 10.1007/s00330-017-5238-5. Epub 2018 Feb 5. Eur Radiol. 2018. PMID: 29404773
-
Dual-energy CT after radiofrequency ablation of liver, kidney, and lung lesions: a review of features.Insights Imaging. 2015 Jun;6(3):363-79. doi: 10.1007/s13244-015-0408-y. Epub 2015 May 5. Insights Imaging. 2015. PMID: 25941033 Free PMC article.
-
Value of spectral detector computed tomography for the early assessment of technique efficacy after microwave ablation of hepatocellular carcinoma.PLoS One. 2021 Jun 15;16(6):e0252678. doi: 10.1371/journal.pone.0252678. eCollection 2021. PLoS One. 2021. PMID: 34129650 Free PMC article.
References
-
- Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol 2009;82:908–15 - PubMed
-
- Montella L, Addeo R, Caraglia M, Faiola V, Guarrasi R, Vincenzi B, et al. Vascular endothelial growth factor monitoring in advanced hepatocellular carcinoma patients treated with radiofrequency ablation plus octreotide: a single center experience. Oncol Rep 2008;20:385–90 - PubMed
-
- Choi H, Loyer EM, DuBrow RA, Kaur H, David CL, Huang S, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics 2001;21:S41–54 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

