Disease duration and the integrity of the nigrostriatal system in Parkinson's disease
- PMID: 23884810
- PMCID: PMC3722357
- DOI: 10.1093/brain/awt192
Disease duration and the integrity of the nigrostriatal system in Parkinson's disease
Abstract
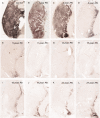
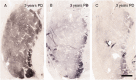
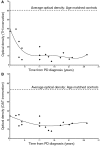
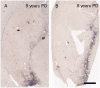
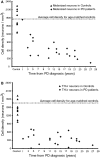
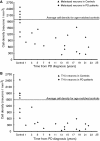
The pace of nigrostriatal degeneration, both with regards to striatal denervation and loss of melanin and tyrosine hydroxylase-positive neurons, is poorly understood especially early in the Parkinson's disease process. This study investigated the extent of nigrostriatal degeneration in patients with Parkinson's disease at different disease durations from time of diagnosis. Brains of patients with Parkinson's disease (n=28) with post-diagnostic intervals of 1-27 years and normal elderly control subjects (n=9) were examined. Sections of the post-commissural putamen and substantia nigra pars compacta were processed for tyrosine hydroxylase and dopamine transporter immunohistochemistry. The post-commissural putamen was selected due to tissue availability and the fact that dopamine loss in this region is associated with motor disability in Parkinson's disease. Quantitative assessments of putaminal dopaminergic fibre density and stereological estimates of the number of melanin-containing and tyrosine hydroxylase-immunoreactive neurons in the substantia nigra pars compacta (both in total and in subregions) were performed by blinded investigators in cases where suitable material was available (n=17). Dopaminergic markers in the dorsal putamen showed a modest loss at 1 year after diagnosis in the single case available for study. There was variable (moderate to marked) loss, at 3 years. At 4 years post-diagnosis and thereafter, there was virtually complete loss of staining in the dorsal putamen with only an occasional abnormal dopaminergic fibre detected. In the substantia nigra pars compacta, there was a 50-90% loss of tyrosine hydroxylase-positive neurons from the earliest time points studied with only marginal additional loss thereafter. There was only a ∼10% loss of melanized neurons in the one case evaluated 1 year post-diagnosis, and variable (30 to 60%) loss during the first several years post-diagnosis with more gradual and subtle loss in the second decade. At all time points, there were more melanin-containing than tyrosine hydroxylase-positive cells. Loss of dopaminergic markers in the dorsal putamen occurs rapidly and is virtually complete by 4 years post-diagnosis. Loss of melanized nigral neurons lags behind the loss of dopamine markers. These findings have important implications for understanding the nature of Parkinson's disease neurodegeneration and for studies of putative neuroprotective/restorative therapies.
Keywords: Parkinsons disease; human brain; morphometry; neuroscience; substantia nigra.
Figures



References
-
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. - PubMed
-
- Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, Kordower JH. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

