Influence of infection during pregnancy on fetal development
- PMID: 23884862
- PMCID: PMC4060827
- DOI: 10.1530/REP-13-0232
Influence of infection during pregnancy on fetal development
Abstract
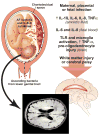
Infection by bacteria, viruses, and parasites may lead to fetal death, organ injury, or limited sequelae depending on the pathogen. Here, we consider the role of infection during pregnancy in fetal development including placental development and function, which can lead to fetal growth restriction. The classical group of teratogenic pathogens is referred to as 'TORCH' (Toxoplasma gondii, others like Treponema pallidum, rubella virus, cytomegalovirus, and herpes simplex virus) but should include a much broader group of pathogens including Parvovirus B19, Varicella zoster virus, and Plasmodium falciparum to name a few. In this review, we describe the influence of different infections in utero on fetal development and the short- and long-term outcomes for the neonate. In some cases, the mechanisms used by these pathogens to disrupt fetal development are well known. Bacterial infection of the developing fetal lungs and brain begins with an inflammatory cascade resulting in cytokine injury and oxidative stress. For some pathogens like P. falciparum, the mechanisms involve oxidative stress and apoptosis to disrupt placental and fetal growth. An in utero infection may also affect the long-term health of the infant; in many cases, a viral infection in utero increases the risk of developing type 1 diabetes in childhood. Understanding the varied mechanisms employed by these pathogens may enable therapies to attenuate changes in fetal development, decrease preterm birth, and improve survival.
Conflict of interest statement
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the reported research.
Figures


References
-
- Egli GE, Newton M. The transport of carbon particles in the human female reproductive tract. Fertil Steril. 1961 Mar-Apr;12:151–155. - PubMed
-
- Cotch MF, Pastorek JG, 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997 Jul;24(6):353–360. - PubMed
-
- Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005 Nov 3;353(18):1899–1911. - PubMed
-
- Menard JP, Mazouni C, Salem-Cherif I, et al. High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor. Obstet Gynecol. 2010 Jan;115(1):134–140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

