Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice
- PMID: 23885013
- PMCID: PMC3776868
- DOI: 10.1210/en.2013-1289
Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice
Abstract
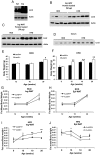
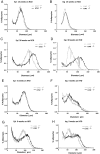
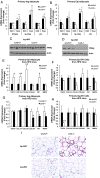
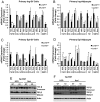
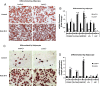
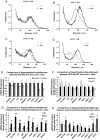
Lipocalin 2 (Lcn2) has previously been characterized as an adipokine/cytokine playing a role in glucose and lipid homeostasis. In this study, we investigate the role of Lcn2 in adipose tissue remodeling during high-fat diet (HFD)-induced obesity. We find that Lcn2 protein is highly abundant selectively in inguinal adipose tissue. During 16 weeks of HFD feeding, the inguinal fat depot expanded continuously, whereas the expansion of the epididymal fat depot was reduced in both wild-type (WT) and Lcn2(-/-) mice. Interestingly, the depot-specific effect of HFD on fat mass was exacerbated and appeared more pronounced and faster in Lcn2(-/-) mice than in WT mice. In Lcn2(-/-) mice, adipocyte hypertrophy in both inguinal and epididymal adipose tissue was more profoundly induced by age and HFD when compared with WT mice. The expression of peroxisome proliferator-activated receptor-γ protein was significantly down-regulated, whereas the gene expression of extracellular matrix proteins was up-regulated selectively in epididymal adipocytes of Lcn2(-/-) mice. Consistent with these observations, collagen deposition was selectively higher in the epididymal, but not in the inguinal adipose depot of Lcn2(-/-) mice. Administration of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone (Rosi) restored adipogenic gene expression. However, Lcn2 deficiency did not alter the responsiveness of adipose tissue to Rosi effects on the extracellular matrix expression. Rosi treatment led to the further enlargement of adipocytes with improved metabolic activity in Lcn2(-/-) mice, which may be associated with a more pronounced effect of Rosi treatment in reducing TGF-β in Lcn2(-/-) adipose tissue. Consistent with these in vivo observations, Lcn2 deficiency reduces the adipocyte differentiation capacity of stromal-vascular cells isolated from HFD-fed mice in these cells. Herein Rosi treatment was again able to stimulate adipocyte differentiation to a similar extent in WT and Lcn2(-/-) inguinal and epididymal stromal-vascular cells. Thus, combined, our data indicate that Lcn2 has a depot-specific role in HFD-induced adipose tissue remodeling.
Figures
Similar articles
-
Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure.FASEB J. 2011 Feb;25(2):754-64. doi: 10.1096/fj.10-165175. Epub 2010 Oct 25. FASEB J. 2011. PMID: 20974668 Free PMC article.
-
A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance.Am J Physiol Endocrinol Metab. 2011 Nov;301(5):E825-35. doi: 10.1152/ajpendo.00147.2011. Epub 2011 Jul 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21771968 Free PMC article.
-
Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism.J Biol Chem. 2014 Aug 8;289(32):22063-77. doi: 10.1074/jbc.M114.559104. Epub 2014 Jun 10. J Biol Chem. 2014. PMID: 24917675 Free PMC article.
-
White adipose tissue dysfunction in obesity and aging.Biochem Pharmacol. 2021 Oct;192:114723. doi: 10.1016/j.bcp.2021.114723. Epub 2021 Aug 5. Biochem Pharmacol. 2021. PMID: 34364887 Review.
-
The sexual dimorphism of obesity.Mol Cell Endocrinol. 2015 Feb 15;402:113-9. doi: 10.1016/j.mce.2014.11.029. Epub 2015 Jan 8. Mol Cell Endocrinol. 2015. PMID: 25578600 Free PMC article. Review.
Cited by
-
The Impact of Aging on Adipose Function and Adipokine Synthesis.Front Endocrinol (Lausanne). 2019 Mar 11;10:137. doi: 10.3389/fendo.2019.00137. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30915034 Free PMC article. Review.
-
Commentary: Mammokine directs beige adipocytes to reserve energy for milk production in breast.Acta Pharm Sin B. 2024 Mar;14(3):1472-1476. doi: 10.1016/j.apsb.2023.11.031. Epub 2023 Dec 4. Acta Pharm Sin B. 2024. PMID: 38486985 Free PMC article. No abstract available.
-
Depot-Dependent Impact of Time-Restricted Feeding on Adipose Tissue Metabolism in High Fat Diet-Induced Obese Male Mice.Nutrients. 2023 Jan 3;15(1):238. doi: 10.3390/nu15010238. Nutrients. 2023. PMID: 36615895 Free PMC article.
-
Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes.PLoS One. 2014 May 12;9(5):e96997. doi: 10.1371/journal.pone.0096997. eCollection 2014. PLoS One. 2014. PMID: 24818605 Free PMC article.
-
Crosstalk Between Mast Cells and Adipocytes in Physiologic and Pathologic Conditions.Clin Rev Allergy Immunol. 2020 Jun;58(3):388-400. doi: 10.1007/s12016-020-08785-7. Clin Rev Allergy Immunol. 2020. PMID: 32215785 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

