Hsc70 negatively regulates epithelial sodium channel trafficking at multiple sites in epithelial cells
- PMID: 23885065
- PMCID: PMC3798670
- DOI: 10.1152/ajpcell.00059.2013
Hsc70 negatively regulates epithelial sodium channel trafficking at multiple sites in epithelial cells
Abstract
The epithelial sodium channel (ENaC) plays an important role in homeostasis of blood pressure and of the airway surface liquid, and excess function of ENaC results in refractory hypertension (in Liddle's syndrome) and impaired mucociliary clearance (in cystic fibrosis). The regulation of ENaC by molecular chaperones, such as the 70-kDa heat shock protein Hsc70, is not completely understood. Our previously published data suggest that Hsc70 negatively affects ENaC activity and surface expression in Xenopus oocytes; here we investigate the mechanism by which Hsc70 acts on ENaC in epithelial cells. In Madin-Darby canine kidney cells stably expressing epitope-tagged αβγ-ENaC and with tetracycline-inducible overexpression of Hsc70, treatment with 5 μg/ml doxycycline increased total Hsc70 expression 20%. This increase in Hsc70 expression led to a decrease in ENaC activity and surface expression that corresponded to an increased rate of functional ENaC retrieval from the cell surface. In addition, Hsc70 overexpression decreased the association of newly synthesized ENaC subunits. These data support the hypothesis that Hsc70 inhibits ENaC functional expression at the apical surface of epithelia by regulating ENaC biogenesis and ENaC trafficking at the cell surface.
Keywords: ENaC; chaperone; epithelia; heat shock protein; trafficking.
Figures






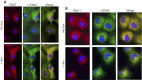


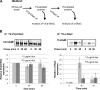
References
-
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem 272: 9002–9010, 1997 - PubMed
-
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007 - PubMed
-
- Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem 282: 37885–37893, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

