Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques
- PMID: 23885083
- PMCID: PMC3807376
- DOI: 10.1128/JVI.00049-13
Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques
Abstract
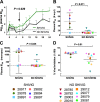
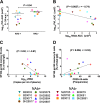
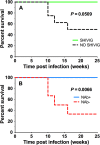
Simian-human immunodeficiency virus (SHIV) models for human immunodeficiency virus (HIV) infection have been widely used in passive studies with HIV neutralizing antibodies (NAbs) to test for protection against infection. However, because SHIV-infected adult macaques often rapidly control plasma viremia and any resulting pathogenesis is minor, the model has been unsuitable for studying the impact of antibodies on pathogenesis in infected animals. We found that SHIVSF162P3 infection in 1-month-old rhesus macaques not only results in high persistent plasma viremia but also leads to very rapid disease progression within 12 to 16 weeks. In this model, passive transfer of high doses of neutralizing IgG (SHIVIG) prevents infection. Here, we show that at lower doses, SHIVIG reduces both plasma and peripheral blood mononuclear cell (PBMC)-associated viremia and mitigates pathogenesis in infected animals. Moreover, production of endogenous NAbs correlated with lower set-point viremia and 100% survival of infected animals. New SHIV models are needed to investigate whether passively transferred antibodies or antibodies elicited by vaccination that fall short of providing sterilizing immunity impact disease progression or influence immune responses. The 1-month-old rhesus macaque SHIV model of infection provides a new tool to investigate the effects of antibodies on viral replication and clearance, mechanisms of B cell maintenance, and the induction of adaptive immunity in disease progression.
Figures





References
-
- Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840 - PMC - PubMed
-
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312 - PubMed
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PWHI, Sawyer LSW, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Stiehm ER, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF., III 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

