Adjudin disrupts spermatogenesis by targeting drug transporters: Lesson from the breast cancer resistance protein (BCRP)
- PMID: 23885306
- PMCID: PMC3710224
- DOI: 10.4161/spmg.24993
Adjudin disrupts spermatogenesis by targeting drug transporters: Lesson from the breast cancer resistance protein (BCRP)
Abstract
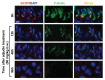
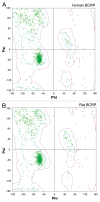
For non-hormonal male contraceptives that exert their effects in the testis locally instead of via the hypothalamic-pituitary-testicular axis, such as adjudin that disrupts germ cell adhesion, a major hurdle in their development is to improve their bioavailability so that they can be efficiently delivered to the seminiferous epithelium by transporting across the blood-testis barrier (BTB). If this can be done, it would widen the gap between their efficacy and general toxicity. However, Sertoli cells that constitute the BTB, peritubular myoid cells in the tunica propria, germ cells at different stages of their development, as well as endothelial cells that constitute the microvessels in the interstitium are all equipped with multiple drug transporters, most notably efflux drug transporters, such as P-glycoprotein, multidrug resistance-related protein 1 (MRP1) and breast cancer resistance protein (BCRP) that can actively prevent drugs (e.g., adjudin) from entering the seminiferous epithelium to exert their effects. Recent studies have shown that BCRP is highly expressed by endothelial cells of the microvessels in the interstitium in the testis and also peritubular myoid cells in tunica propria even though it is absent from Sertoli cells at the site of the BTB. Furthermore, BCRP is also expressed spatiotemporally by Sertoli cells and step 19 spermatids in the rat testis and stage-specifically, limiting to stage VII‒VIII of the epithelial cycle, and restricted to the apical ectoplasmic specialization [apical ES, a testis-specific F-actin-rich adherens junction (AJ)]. Interestingly, adjudin was recently shown to be capable of downregulating BCRP expression at the apical ES. In this Opinion article, we critically discuss the latest findings on BCRP; in particular, we provide some findings utilizing molecular modeling to define the interacting domains of BCRP with adjudin. Based on this information, it is hoped that the next generation of adjudin analogs to be synthesized can improve their efficacy in downregulating BCRP and perhaps other drug efflux transporters in the testis to improve their efficacy to traverse the BTB by modifying their interacting domains.
Keywords: Bcrp; adjudin; ectoplasmic specialization; male contraception; spermatogenesis; testis.
Figures



Similar articles
-
Effective Delivery of Male Contraceptives Behind the Blood-Testis Barrier (BTB) - Lesson from Adjudin.Curr Med Chem. 2016;23(7):701-13. doi: 10.2174/0929867323666160112122724. Curr Med Chem. 2016. PMID: 26758796 Free PMC article. Review.
-
Interaction of oligomeric breast cancer resistant protein (BCRP) with adjudin: a male contraceptive with anti-cancer activity.Curr Mol Pharmacol. 2014;7(2):147-53. doi: 10.2174/1874467208666150126154049. Curr Mol Pharmacol. 2014. PMID: 25620224 Free PMC article. Review.
-
Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis.Am J Physiol Endocrinol Metab. 2013 Apr 1;304(7):E757-69. doi: 10.1152/ajpendo.00645.2012. Epub 2013 Feb 12. Am J Physiol Endocrinol Metab. 2013. PMID: 23403943 Free PMC article.
-
Breast cancer resistance protein (Bcrp) and the testis--an unexpected turn of events.Asian J Androl. 2013 Jul;15(4):455-60. doi: 10.1038/aja.2013.24. Epub 2013 May 13. Asian J Androl. 2013. PMID: 23665760 Free PMC article. Review.
-
Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3.Spermatogenesis. 2011 Oct;1(4):291-297. doi: 10.4161/spmg.1.4.18393. Epub 2011 Oct 1. Spermatogenesis. 2011. PMID: 22332112 Free PMC article.
Cited by
-
Use of Molecular Modeling to Study Spermatogenesis: An Overview Using Proteins in Sertoli Cells.Adv Exp Med Biol. 2021;1288:205-214. doi: 10.1007/978-3-030-77779-1_10. Adv Exp Med Biol. 2021. PMID: 34453738 Review.
-
Transepithelial transport across the blood-testis barrier.Reproduction. 2018 Dec;156(6):R187-R194. doi: 10.1530/REP-18-0338. Reproduction. 2018. PMID: 30328342 Free PMC article. Review.
-
Effective Delivery of Male Contraceptives Behind the Blood-Testis Barrier (BTB) - Lesson from Adjudin.Curr Med Chem. 2016;23(7):701-13. doi: 10.2174/0929867323666160112122724. Curr Med Chem. 2016. PMID: 26758796 Free PMC article. Review.
-
Improved scFv Anti-LOX-1 Binding Activity by Fusion with LOX-1-Binding Peptides.Biomed Res Int. 2017;2017:8946935. doi: 10.1155/2017/8946935. Epub 2017 Sep 28. Biomed Res Int. 2017. PMID: 29094051 Free PMC article.
-
Interaction of oligomeric breast cancer resistant protein (BCRP) with adjudin: a male contraceptive with anti-cancer activity.Curr Mol Pharmacol. 2014;7(2):147-53. doi: 10.2174/1874467208666150126154049. Curr Mol Pharmacol. 2014. PMID: 25620224 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
