Angiogenic endothelial cell invasion into fibrin is stimulated by proliferating smooth muscle cells
- PMID: 23886898
- PMCID: PMC3849341
- DOI: 10.1016/j.mvr.2013.06.012
Angiogenic endothelial cell invasion into fibrin is stimulated by proliferating smooth muscle cells
Abstract
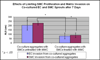
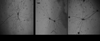
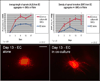
These studies aimed to determine the effect of smooth muscle cells (SMCs) on angiogenic behavior of endothelial cells (ECs) within fibrin hydrogels, an extracellular matrix (ECM) commonly used in tissue engineering. We developed a 3-D, fibrin-based co-culture assay of angiogenesis consisting of aggregates of SMCs with ECs seeded onto the aggregates' surface. Using digital fluorescence micrography, EC matrix invasion was quantified by average length of sprouts (ALS) and density of sprout formation (DSF). We demonstrated that ECs and SMCs co-invade into the ECM in close proximity to one another. ECs that were co-cultured with SMCs demonstrated increased invasion compared to ECs that were cultured alone at all time points. At Day 19, the ALS of ECs in co-culture was 327+/-58μm versus 70+/-11μm of ECs cultured alone (p=.01). The DSF of co-cultured ECs was also significantly greater than that of ECs cultured alone (p=.007 on Day 19). This appeared to be a function of both increased EC invasion as well as improved persistence of EC sprout networks. At 7days, ECs in co-culture with proliferation-inhibited SMCs previously treated with Mitomycin-C (MMC) demonstrated significantly attenuated sprouting compared to ECs co-cultured with SMCs that were untreated with MMC (82+/-14μm versus 205+/-32μm; p<.05). In assays in which multiple co-culture aggregates were cultured within a single hydrogel, we observed directional invasion of sprouts preferentially towards the other aggregates within the hydrogel. In co-culture assays without early EC/SMC contact, the ALS of ECs cultured in the presence of SMCs was significantly greater than those cultured in the absence of SMCs by Day 3 (320+/-21μm versus 187+/-16μm; p<.005). We conclude that SMCs augment EC matrix invasion into 3-D fibrin hydrogels, at least in part resulting from SMC proliferative and invasive activities. Directed invasion between co-culture aggregates and augmented angiogenesis in the absence of early contact suggests a paracrine mechanism for the observed results.
© 2013 Elsevier Inc. All rights reserved.
Figures






References
-
- Brewster LP, Brey EM, Tassiopoulos AK, Xue L, Maddox E, Armistead D, Burgess WH, Greisler HP. "Heparin-independent mitogenicity in an endothelial and smooth muscle cell chimeric growth factor (S130K-HBGAM)". Am J Surg. 2004;188(5):575–579. - PubMed
-
- Brey EM, McIntire LV, Johnston CM, Reece GP, Patrick CW., Jr. "Three-dimensional, quantitative analysis of desmin and smooth muscle alpha actin expression during angiogenesis". Annals of Biomedical Engineering. 2004;32(8):1100. - PubMed
-
- Chon JH, Wang HS, Chaikof EL. "Role of fibronectin and sulfated proteoglycans in endothelial cell migration on a cultured smooth muscle layer". The Journal of surgical research. 1997;72(1):53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

