From structure to function: mitochondrial morphology, motion and shaping in vascular smooth muscle
- PMID: 23887139
- PMCID: PMC3884171
- DOI: 10.1159/000353883
From structure to function: mitochondrial morphology, motion and shaping in vascular smooth muscle
Abstract
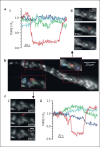
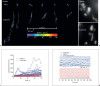
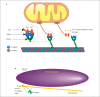
The diversity of mitochondrial arrangements, which arise from the organelle being static or moving, or fusing and dividing in a dynamically reshaping network, is only beginning to be appreciated. While significant progress has been made in understanding the proteins that reorganise mitochondria, the physiological significance of the various arrangements is poorly understood. The lack of understanding may occur partly because mitochondrial morphology is studied most often in cultured cells. The simple anatomy of cultured cells presents an attractive model for visualizing mitochondrial behaviour but contrasts with the complexity of native cells in which elaborate mitochondrial movements and morphologies may not occur. Mitochondrial changes may take place in native cells (in response to stress and proliferation), but over a slow time-course and the cellular function contributed is unclear. To determine the role mitochondrial arrangements play in cell function, a crucial first step is characterisation of the interactions among mitochondrial components. Three aspects of mitochondrial behaviour are described in this review: (1) morphology, (2) motion and (3) rapid shape changes. The proposed physiological roles to which various mitochondrial arrangements contribute and difficulties in interpreting some of the physiological conclusions are also outlined.
© 2013 S. Karger AG, Basel.
Figures

References
-
- Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. - PubMed
-
- Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42:447–466. - PubMed
-
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. - PubMed
-
- Tandler B, Hoppel CL. Studies on giant mitochondria. Ann NY Acad Sci. 1986;488:65–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

