A novel C5a-neutralizing mirror-image (l-)aptamer prevents organ failure and improves survival in experimental sepsis
- PMID: 23887360
- PMCID: PMC3863792
- DOI: 10.1038/mt.2013.178
A novel C5a-neutralizing mirror-image (l-)aptamer prevents organ failure and improves survival in experimental sepsis
Abstract
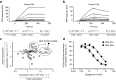
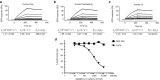
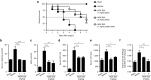
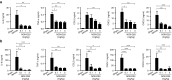
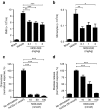
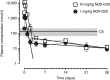
Complement factor C5a is a potent proinflammatory mediator that contributes to the pathogenesis of numerous inflammatory diseases. Here, we describe the discovery of NOX-D20, a PEGylated biostable mirror-image mixed (l-)RNA/DNA aptamer (Spiegelmer) that binds to mouse and human C5a with picomolar affinity. In vitro, NOX-D20 inhibited C5a-induced chemotaxis of a CD88-expressing cell line and efficiently antagonized the activation of primary human polymorphonuclear leukocytes (PMN) by C5a. Binding of NOX-D20 to the C5a moiety of human C5 did not interfere with the formation of the terminal membrane attack complex (MAC). In sepsis, for which a specific interventional therapy is currently lacking, complement activation and elevated levels of C5a are suggested to contribute to multiorgan failure and mortality. In the model of polymicrobial sepsis induced by cecal ligation and puncture (CLP), NOX-D20 attenuated inflammation and organ damage, prevented the breakdown of the vascular endothelial barrier, and improved survival. Our study suggests NOX-D20 as a new therapeutic candidate for the treatment of sepsis.
Figures

Similar articles
-
Intravitreal inhibition of complement C5a reduces choroidal neovascularization in mice.Graefes Arch Clin Exp Ophthalmol. 2015 Oct;253(10):1695-704. doi: 10.1007/s00417-015-3041-z. Epub 2015 May 16. Graefes Arch Clin Exp Ophthalmol. 2015. PMID: 25981118
-
C5a, a therapeutic target in sepsis.Recent Pat Antiinfect Drug Discov. 2006 Jan;1(1):57-65. doi: 10.2174/157489106775244091. Recent Pat Antiinfect Drug Discov. 2006. PMID: 18221134 Review.
-
Carboxypeptidase B2 deficiency reveals opposite effects of complement C3a and C5a in a murine polymicrobial sepsis model.J Thromb Haemost. 2015 Jun;13(6):1090-102. doi: 10.1111/jth.12956. Epub 2015 May 10. J Thromb Haemost. 2015. PMID: 25851247 Free PMC article.
-
Neutralizing Complement C5a Protects Mice with Pneumococcal Pulmonary Sepsis.Anesthesiology. 2020 Apr;132(4):795-807. doi: 10.1097/ALN.0000000000003149. Anesthesiology. 2020. PMID: 32101978
-
Role of C5 activation products in sepsis.ScientificWorldJournal. 2010 Dec 14;10:2395-402. doi: 10.1100/tsw.2010.216. ScientificWorldJournal. 2010. PMID: 21170490 Free PMC article. Review.
Cited by
-
Chemically modified aptamers for improving binding affinity to the target proteins via enhanced non-covalent bonding.Front Cell Dev Biol. 2023 Feb 23;11:1091809. doi: 10.3389/fcell.2023.1091809. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910146 Free PMC article. Review.
-
Targeting Complement Pathways in Polytrauma- and Sepsis-Induced Multiple-Organ Dysfunction.Front Immunol. 2019 Mar 21;10:543. doi: 10.3389/fimmu.2019.00543. eCollection 2019. Front Immunol. 2019. PMID: 30949180 Free PMC article. Review.
-
Anti-inflammatory interventions-what has worked, not worked, and what may work in the future.Transl Res. 2016 Jan;167(1):1-6. doi: 10.1016/j.trsl.2015.08.003. Epub 2015 Aug 14. Transl Res. 2016. PMID: 26323016 Free PMC article.
-
Protective Effects of a Dihydrodiazepine Against Endotoxin Shock Through Suppression of TLR4/NF-κB/IRF3 Signaling Pathways.Inflammation. 2025 Aug;48(4):1863-1878. doi: 10.1007/s10753-024-02160-w. Epub 2024 Oct 14. Inflammation. 2025. PMID: 39400777
-
Be on Target: Strategies of Targeting Alternative and Lectin Pathway Components in Complement-Mediated Diseases.Front Immunol. 2018 Aug 8;9:1851. doi: 10.3389/fimmu.2018.01851. eCollection 2018. Front Immunol. 2018. PMID: 30135690 Free PMC article. Review.
References
-
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. - PubMed
-
- Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012;44:205–217. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

