How can plant DNA viruses evade siRNA-directed DNA methylation and silencing?
- PMID: 23887650
- PMCID: PMC3759858
- DOI: 10.3390/ijms140815233
How can plant DNA viruses evade siRNA-directed DNA methylation and silencing?
Abstract
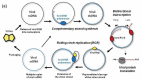
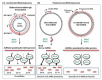
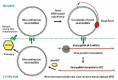
Plants infected with DNA viruses produce massive quantities of virus-derived, 24-nucleotide short interfering RNAs (siRNAs), which can potentially direct viral DNA methylation and transcriptional silencing. However, growing evidence indicates that the circular double-stranded DNA accumulating in the nucleus for Pol II-mediated transcription of viral genes is not methylated. Hence, DNA viruses most likely evade or suppress RNA-directed DNA methylation. This review describes the specialized mechanisms of replication and silencing evasion evolved by geminiviruses and pararetoviruses, which rescue viral DNA from repressive methylation and interfere with transcriptional and post-transcriptional silencing of viral genes.
Figures


References
-
- Gronenborn B. Nanoviruses: Genome organisation and protein function. Vet. Microbiol. 2004;98:103–109. - PubMed
-
- Llave C. Virus-derived small interfering RNAs at the core of plant-virus interactions. Trends Plant Sci. 2010;15:701–707. - PubMed
-
- Pantaleo V. Plant RNA silencing in viral defence. Adv. Exp. Med. Biol. 2011;722:39–58. - PubMed
-
- Rajeswaran R., Pooggin M.M. Role of Virus-Derived Small RNAs in Plant Antiviral Defense: Insights from DNA Viruses. In: Sunkar R., editor. MicroRNAs in Plant Development and Stress Response. Springer-Verlag; Berlin Heidelberg, Germany: 2012. pp. 261–289.
-
- Szittya G., Burgyán J. RNA interference-mediated intrinsic antiviral immunity in plants. Curr. Top. Microbiol. Immunol. 2013;371:153–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

