A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans
- PMID: 23888036
- PMCID: PMC3826785
- DOI: 10.1126/science.1236231
A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans
Abstract
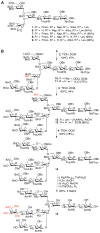
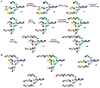
A systematic, efficient means of producing diverse libraries of asymmetrically branched N-glycans is needed to investigate the specificities and biology of glycan-binding proteins. To that end, we describe a core pentasaccharide that at potential branching positions is modified by orthogonal protecting groups to allow selective attachment of specific saccharide moieties by chemical glycosylation. The appendages were selected so that the antenna of the resulting deprotected compounds could be selectively extended by glycosyltransferases to give libraries of asymmetrical multi-antennary glycans. The power of the methodology was demonstrated by the preparation of a series of complex oligosaccharides that were printed as microarrays and screened for binding to lectins and influenza-virus hemagglutinins, which showed that recognition is modulated by presentation of minimal epitopes in the context of complex N-glycans.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
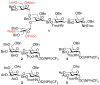
 ); D-galactose (Gal,
); D-galactose (Gal,  ); N-acetyl-D-glucosamine
(GlcNAc,
); N-acetyl-D-glucosamine
(GlcNAc,  ); D-mannose (Man,
); D-mannose (Man,
 ); L-fucose (Fuc,
); L-fucose (Fuc,
 ).
).
Comment in
-
Chemistry. A path to complex carbohydrates.Science. 2013 Jul 26;341(6144):357-8. doi: 10.1126/science.1241788. Science. 2013. PMID: 23888030 No abstract available.
-
Biochemistry: Constructing complicated carbohydrates.Nat Methods. 2013 Oct;10(10):932. doi: 10.1038/nmeth.2677. Nat Methods. 2013. PMID: 24161973 No abstract available.
References
-
- Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855. - PubMed
-
- Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

