Effect of 6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride (Voluven®) on complications after subarachnoid hemorrhage: a retrospective analysis
- PMID: 23888282
- PMCID: PMC3717154
- DOI: 10.1186/2193-1801-2-314
Effect of 6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride (Voluven®) on complications after subarachnoid hemorrhage: a retrospective analysis
Abstract
Background: 6% Hydroxyethyl Starch 130/0.4 in 0.9% Sodium Chloride (Voluven®; 6% HES 130/0.4) is a colloid often used for fluid resuscitation in patients with subarachnoid hemorrhage (SAH), despite a lack of safety data for this use. The purpose of our study was to evaluate the effect of 6% HES 130/0.4 on major complications associated with SAH.
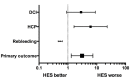
Methods: Medical records of all patients presenting between May 2010 and September 2012 with aneurysmal SAH were analyzed. Patients were divided in two groups based on the administration of 6% HES 130/0.4; HES group (n=57) and Non-HES group (n=72). The primary outcome included a composite of three major complications associated with SAH: Delayed Cerebral Ischemia (DCI), Hydrocephalus (HCP) requiring cerebrospinal fluid (CSF) shunting, and Rebleeding.
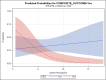
Results: The study groups were similar with respect to most characteristics except the incidences of hypertension, ischemic heart disease, Fisher grade and lowest hemoglobin during stay. The odds of developing the primary composite outcome was higher in the HES group [OR= 3.1(1.30-7.36), p=0.01]. The patients in the HES group had a significantly longer median duration of hospital (19 vs 14 days) and Neurointensive Care Unit stay (14 vs 10 days) compared to the Non HES group.
Conclusion: We observed increased complications after SAH with 6% HES 130/0.4 (Voluven®) administration. An adequately powered prospective randomized controlled trial into the safety of 6% HES 130/0.4 in this patient population is warranted.
Keywords: 6% HES 130/0.4; Delayed cerebral ischemia; Fluid therapy; Hydrocephalus; Hydroxyethyl starch; Mortality; Rebleeding; Subarachnoid hemorrhage; Voluven.
Figures
References
-
- Anonymous . HIGHLIGHTS OF PRESCRIBING INFORMATION: Voluven® (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection) 2013.
-
- Anonymous HES 130/0,4/9:1 effect on intracranial pressure, cerebral blood flow, oxygenation and cerebral vasospasm. Anesteziol Reanimatol. 2012;4:63–68. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous