Epigenetic regulation of EMT: the Snail story
- PMID: 23888971
- PMCID: PMC4005722
- DOI: 10.2174/13816128113199990512
Epigenetic regulation of EMT: the Snail story
Abstract
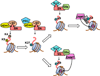
While the epithelial-mesenchymal transition (EMT) plays a fundamental role during development, its deregulation can adversely promote tumor metastasis. The phenotypic and cellular plasticity of EMT indicates that it is subject to epigenetic regulation. A hallmark of EMT is E-cadherin suppression. In this review, we try to embrace recent findings on the transcription factor Snail-mediated epigenetic silencing of E-cadherin. Our studies as well as those of others independently demonstrated that Snail can recruit various epigenetic machineries to the E-cadherin promoter. Based on these results, we propose a model of epigenetic regulation of EMT governed by Snail. Briefly, recruitment of the LSD1/HDAC complex by Snail facilitates histone H3K4 demethylation and H3/H4 deacetylation. Histone deacetylation may promote subsequent recruitment of PRC2 to methylate H3K27, while H3K4 demethylation favors the association of H3K9 methyltransferases G9a and Suv39H1. Finally, DNA methyltransferases (DNMTs) can be recruited to the promoter area in a G9a/Suv39H1-dependent manner. Together, these chromatin-modifying enzymes function in a Snail-mediated, highly orchestrated fashion to suppress E-cadherin. Disruption of the connection between Snail and these epigenetic machineries may represent an efficient strategy for the treatment of EMT-related diseases, including tumor metastasis.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists
References
-
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials