Enantioselective α-alkenylation of aldehydes with boronic acids via the synergistic combination of copper(II) and amine catalysis
- PMID: 23889497
- PMCID: PMC3785792
- DOI: 10.1021/ja406356c
Enantioselective α-alkenylation of aldehydes with boronic acids via the synergistic combination of copper(II) and amine catalysis
Abstract
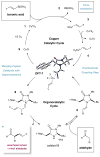
The enantioselective α-alkenylation of aldehydes has been accomplished using boronic acids via the synergistic combination of copper and chiral amine catalysis. The merger of two highly utilized and robust catalytic systems has allowed for the development of a mild and operationally trivial protocol for the direct formation of α-formyl olefins employing common building blocks for organic synthesis.
Figures
References
-
-
For a review on synergistic catalysis, see: Allen AE, MacMillan DWC. Chem Sci. 2012;3:633.For reviews on dual catalysis, see: Shao Z, Zhang H. Chem Soc Rev. 2009;38:2745.Zhong C, Shi X. Eur J Org Chem. 2010:2999.Patil NT, Shinde VS, Gajula B. Org Biomol Chem. 2012;10:211.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources