Obligatory role for the immediate early gene NARP in critical period plasticity
- PMID: 23889936
- PMCID: PMC3804028
- DOI: 10.1016/j.neuron.2013.05.016
Obligatory role for the immediate early gene NARP in critical period plasticity
Abstract
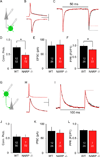
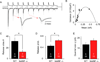
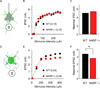
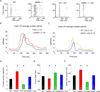
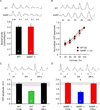
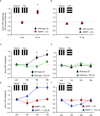
The immediate early gene neuronal activity-regulated pentraxin (NARP) is an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) binding protein that is specifically enriched at excitatory synapses onto fast-spiking parvalbumin-positive interneurons (FS [PV] INs). Here, we show that transgenic deletion of NARP decreases the number of excitatory synaptic inputs onto FS (PV) INs and reduces net excitatory synaptic drive onto FS (PV) INs. Accordingly, the visual cortex of NARP(-/-) mice is hyperexcitable and unable to express ocular dominance plasticity, although many aspects of visual function are unimpaired. Importantly, the number and strength of inhibitory synaptic contacts from FS (PV) INs onto principle neurons in the visual cortex is normal in NARP(-/-) mice, and enhancement of this output recovers the expression of experience-dependent synaptic plasticity. Thus the recruitment of inhibition from FS (PV) INs plays a central role in enabling the critical period for ocular dominance plasticity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. - PubMed
-
- Beste C, Wascher E, Güntürkün O, Dinse HR. Improvement and impairment of visually guided behavior through LTP- and LTD-like exposure-based visual learning. Curr Biol. 2011;21:876–882. - PubMed
-
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

