A biophysically detailed model of neocortical local field potentials predicts the critical role of active membrane currents
- PMID: 23889937
- PMCID: PMC3732581
- DOI: 10.1016/j.neuron.2013.05.023
A biophysically detailed model of neocortical local field potentials predicts the critical role of active membrane currents
Abstract
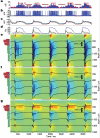
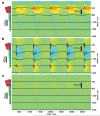
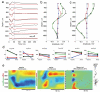
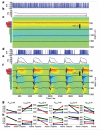
Brain activity generates extracellular voltage fluctuations recorded as local field potentials (LFPs). It is known that the relevant microvariables, the ionic currents across membranes, jointly generate the macrovariables, the extracellular voltage, but neither the detailed biophysical knowledge nor the required computational power have been available to model these processes. We simulated the LFP in a model of the rodent neocortical column composed of >12,000 reconstructed, multicompartmental, and spiking cortical layer 4 and 5 pyramidal neurons and basket cells, including five million dendritic and somatic compartments with voltage- and ion-dependent currents, realistic connectivity, and probabilistic AMPA, NMDA, and GABA synapses. We found that, depending on a number of factors, the LFP reflects local and cross-layer processing. Active currents dominate the generation of LFPs, not synaptic ones. Spike-related currents impact the LFP not only at higher frequencies but below 50 Hz. This work calls for re-evaluating the genesis of LFPs.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Achermann P, Borbély A. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. - PubMed
-
- Anastassiou CA, Perin R, Markram H, Koch C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 2011;14:217–223. - PubMed
-
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J. Neurophysiol. 1999;82:1295–1302. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

