Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced
- PMID: 23889974
- PMCID: PMC3971054
- DOI: 10.1111/jce.12222
Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced
Abstract
Introduction: The traditional description of the Triangle of Dysplasia in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C) predates genetic testing and excludes biventricular phenotypes.
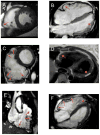
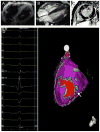
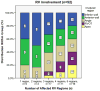
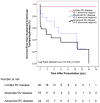
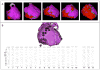
Methods and results: We analyzed Cardiac Magnetic Resonance (CMR) studies of 74 mutation-positive ARVD/C patients for regional abnormalities on a 5-segment RV and 17-segment LV model. The location of electroanatomic endo- and epicardial scar and site of successful VT ablation was recorded in 11 ARVD/C subjects. Among 54/74 (73%) subjects with abnormal CMR, the RV was abnormal in almost all (96%), and 52% had biventricular involvement. Isolated LV abnormalities were uncommon (4%). Dyskinetic basal inferior wall (94%) was the most prevalent RV abnormality, followed by basal anterior wall (87%) dyskinesis. Subepicardial fat infiltration in the posterolateral LV (80%) was the most frequent LV abnormality. Similar to CMR data, voltage maps revealed scar (<0.5 mV) in the RV basal inferior wall (100%), followed by the RV basal anterior wall (64%) and LV posterolateral wall (45%). All 16 RV VTs originated from the basal inferior wall (50%) or basal anterior wall (50%). Of 3 LV VTs, 2 localized to the posterolateral wall. In both modalities, RV apical involvement never occurred in isolation.
Conclusion: Mutation-positive ARVD/C exhibits a previously unrecognized characteristic pattern of disease involving the basal inferior and anterior RV, and the posterolateral LV. The RV apex is only involved in advanced ARVD/C, typically as a part of global RV involvement. These results displace the RV apex from the Triangle of Dysplasia, and provide insights into the pathophysiology of ARVD/C.
Keywords: arrhythmogenic right ventricular dysplasia/cardiomyopathy; electroanatomic mapping; genetics; implantable cardioverter defibrillator; magnetic resonance imaging; phenotype; ventricular tachcardia.
© 2013 Wiley Periodicals, Inc.
Figures
References
-
- Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. - PubMed
-
- Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. - PubMed
-
- Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. - PubMed
-
- Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. - PubMed
-
- Marcus FI, Zareba W, Calkins H, Towbin JA, Basso C, Bluemke DA, Estes NA, 3rd, Picard MH, Sanborn D, Thiene G, Wichter T, Cannom D, Wilber DJ, Scheinman M, Duff H, Daubert J, Talajic M, Krahn A, Sweeney M, Garan H, Sakaguchi S, Lerman BB, Kerr C, Kron J, Steinberg JS, Sherrill D, Gear K, Brown M, Severski P, Polonsky S, McNitt S. Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm. 2009;6:984–992. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

