Inhibiting biofilm formation by Klebsiella pneumoniae B5055 using an iron antagonizing molecule and a bacteriophage
- PMID: 23889975
- PMCID: PMC3726515
- DOI: 10.1186/1471-2180-13-174
Inhibiting biofilm formation by Klebsiella pneumoniae B5055 using an iron antagonizing molecule and a bacteriophage
Abstract
Background: Success of biofilm dwelling bacteria in causing persistent and chronic infections is attributed to their resistance towards antibiotics and immune defences. Free iron is critical for the growth of biofilm associated bacteria. Therefore in the present study, the effect of limiting iron levels by addition of divalent Co[II] ions in combination with a bacteriophage was used for preventing/disrupting Klebsiella pneumoniae biofilms.
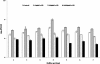
Results: A significantly higher reduction (p < 0.005) in bacterial numbers in the younger as well as older biofilms treated with Co[II] and depolymerase producing phage in combination was observed in comparison to when either of the agents was used alone. The role of phage borne depolymerase was confirmed, as an insignificant eradication of biofilm by non-depolymerase producing bacteriophage in combination with cobalt ions was observed. The results of viable count were further confirmed by visual examination of biofilms.
Conclusion: From the study it can be concluded, that iron antagonizing molecules and bacteriophages can be used as adjunct therapy for preventing biofilm development.
Figures
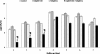
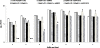
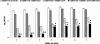

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

