PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling
- PMID: 23890009
- PMCID: PMC4878021
- DOI: 10.1016/j.chembiol.2013.05.010
PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling
Abstract
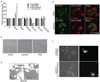
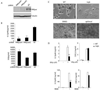
Toll-like receptor (TLR) signaling is a key component of innate immunity. Aberrant TLR activation leads to immune disorders via dysregulation of cytokine production, such as IL-12/IL-23. Herein, we identify and characterize PIKfyve, a lipid kinase, as a critical player in TLR signaling using apilimod as an affinity tool. Apilimod is a potent small molecular inhibitor of IL-12/IL-23 with an unknown target and has been evaluated in clinical trials for patients with Crohn's disease or rheumatoid arthritis. Using a chemical genetic approach, we show that it binds to PIKfyve and blocks its phosphotransferase activity, leading to selective inhibition of IL-12/IL-23p40. Pharmacological or genetic inactivation of PIKfyve is necessary and sufficient for suppression of IL-12/IL-23p40 expression. Thus, we have uncovered a phosphoinositide-mediated regulatory mechanism that controls TLR signaling.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu. Rev Med. 2009;60:97–110. - PubMed
-
- Billich A. Drug evaluation: apilimod, an oral IL-12/IL-23 inhibitor for the treatment of autoimmune diseases and common variable immunodeficiency. IDrugs. 2007;10:53–59. - PubMed
-
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. - PubMed
-
- Kirk CJ, Morris AJ, Shears SB. Peptide Hormone Action: a Practical Approach. Oxford: IRL Press; 1990. pp. 151–158.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

