Phenotypic assays for β-amyloid in mouse embryonic stem cell-derived neurons
- PMID: 23890013
- PMCID: PMC3780781
- DOI: 10.1016/j.chembiol.2013.06.005
Phenotypic assays for β-amyloid in mouse embryonic stem cell-derived neurons
Abstract
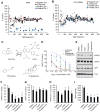
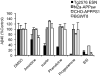
Given the complex nature of Alzheimer's disease (AD), a cell-based model that recapitulates the physiological properties of the target neuronal population would be extremely valuable for discovering improved drug candidates and chemical probes to uncover disease mechanisms. We established phenotypic neuronal assays for the biogenesis and synaptic action of amyloid β peptide (Aβ) based on embryonic stem cell-derived neurons (ESNs). ESNs enriched with pyramidal neurons were robust, scalable, and amenable to a small-molecule screening assay, overcoming the apparent limitations of neuronal models derived from human pluripotent cells. Small-molecule screening of clinical compounds identified four compounds capable of reducing Aβ levels in ESNs derived from the Tg2576 mouse model of AD. Our approach is therefore highly suitable for phenotypic screening in AD drug discovery and has the potential to identify therapeutic candidates with improved efficacy and safety potential.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
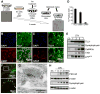
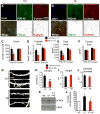
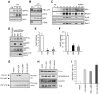

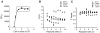
References
-
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. - PubMed
-
- Barker RA. Stem cells and neurodegenerative diseases: where is it all going? Regen Med. 2012;7:26–31. - PubMed
-
- Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde YA. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7:1003–1009. - PubMed
-
- Bibel M, Richter J, Lacroix E, Barde YA. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2:1034–1043. - PubMed
-
- Braginskaya FI, Zorina M, Pal’mina NP, Gaintseva VD, Burlakova EB, Selezneva ND, Kolykhalov IV, Gavrilova SI. Some blood biochemistry parameters during the cholinergic treatment of Alzheimer’s disease. Neurosci Behav Physiol. 2001;31:457–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

