Dendritic-cell-based therapeutic cancer vaccines
- PMID: 23890062
- PMCID: PMC3788678
- DOI: 10.1016/j.immuni.2013.07.004
Dendritic-cell-based therapeutic cancer vaccines
Abstract
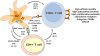
The past decade has seen tremendous developments in novel cancer therapies through the targeting of tumor-cell-intrinsic pathways whose activity is linked to genetic alterations and the targeting of tumor-cell-extrinsic factors, such as growth factors. Furthermore, immunotherapies are entering the clinic at an unprecedented speed after the demonstration that T cells can efficiently reject tumors and that their antitumor activity can be enhanced with antibodies against immune-regulatory molecules (checkpoint blockade). Current immunotherapy strategies include monoclonal antibodies against tumor cells or immune-regulatory molecules, cell-based therapies such as adoptive transfer of ex-vivo-activated T cells and natural killer cells, and cancer vaccines. Herein, we discuss the immunological basis for therapeutic cancer vaccines and how the current understanding of dendritic cell and T cell biology might enable the development of next-generation curative therapies for individuals with cancer.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73:19–29. - PubMed
-
- Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. - PubMed
-
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. - PubMed
-
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

