Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms
- PMID: 23890237
- PMCID: PMC3811070
- DOI: 10.1016/j.cvsm.2013.05.002
Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms
Abstract
Although it is widely appreciated that cats respond differently to certain drugs compared with other companion animal species, the causes of these differences are poorly understood. This article evaluates published evidence for altered drug effects in cats, focusing on pharmacokinetic differences between cats, dogs, and humans, and the molecular mechanisms underlying these differences. More work is needed to better understand drug metabolism and disposition differences in cats, thereby enabling more rational prescribing of existing medications, and the development of safer drugs for this species.
Keywords: Cat; Glucuronidation; Pharmacokinetics; Species differences.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


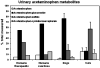
Similar articles
-
Drug therapy in cats: mechanisms and avoidance of adverse drug reactions.J Am Vet Med Assoc. 1990 Apr 15;196(8):1297-305. J Am Vet Med Assoc. 1990. PMID: 2185205 Review.
-
Current concepts on the use of antimicrobials in cats.Vet J. 2009 Jun;180(3):304-16. doi: 10.1016/j.tvjl.2008.01.001. Epub 2008 Mar 7. Vet J. 2009. PMID: 18314361 Review.
-
Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease.J Vet Pharmacol Ther. 2020 Nov;43(6):547-556. doi: 10.1111/jvp.12888. Epub 2020 Jul 12. J Vet Pharmacol Ther. 2020. PMID: 32656792
-
Issues related to the use of canines in toxicologic pathology--issues with pharmacokinetics and metabolism.Toxicol Pathol. 2003 Jan-Feb;31 Suppl:17-24. doi: 10.1080/01926230390174896. Toxicol Pathol. 2003. PMID: 12597427 Review.
-
Out-patient antimicrobial drug use in dogs and cats for new disease events from community companion animal practices in Ontario.Can Vet J. 2012 Mar;53(3):291-8. Can Vet J. 2012. PMID: 22942447 Free PMC article.
Cited by
-
Comparative metabolism as a key driver of wildlife species sensitivity to human and veterinary pharmaceuticals.Philos Trans R Soc Lond B Biol Sci. 2014 Nov 19;369(1656):20130583. doi: 10.1098/rstb.2013.0583. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25405970 Free PMC article. Review.
-
Veterinary Pharmaceutics: An Opportunity for Interprofessional Education in New Zealand?Pharmaceutics. 2017 Jul 26;9(3):25. doi: 10.3390/pharmaceutics9030025. Pharmaceutics. 2017. PMID: 28933730 Free PMC article.
-
Soy isoflavone metabolism in cats compared with other species: urinary metabolite concentrations and glucuronidation by liver microsomes.Xenobiotica. 2016;46(5):406-15. doi: 10.3109/00498254.2015.1086038. Epub 2015 Sep 14. Xenobiotica. 2016. PMID: 26366946 Free PMC article.
-
Safety and efficacy of propyl gallate for all animal species.EFSA J. 2020 Apr 30;18(4):e06069. doi: 10.2903/j.efsa.2020.6069. eCollection 2020 Apr. EFSA J. 2020. PMID: 32874281 Free PMC article.
-
Feline non-regenerative anemia: Diagnostic and treatment recommendations.J Feline Med Surg. 2019 Jul;21(7):615-631. doi: 10.1177/1098612X19856178. J Feline Med Surg. 2019. PMID: 31234748 Free PMC article.
References
-
- Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36(7):1385–1405. - PubMed
-
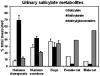
- Parton K, Balmer TV, Boyle J, et al. The pharmacokinetics and effects of intravenously administered carprofen and salicylate on gastrointestinal mucosa and selected biochemical measurements in healthy cats. J Vet Pharmacol Ther. 2000;23(2):73–79. - PubMed
-
- Waters DJ, Bowers LD, Cipolle RJ, et al. Plasma salicylate concentrations in immature dogs following aspirin administration: comparison with adult dogs. J Vet Pharmacol Ther. 1993;16(3):275–282. - PubMed
-
- Greenblatt DJ, Abernethy DR, Boxenbaum HG, et al. Influence of age, gender, and obesity on salicylate kinetics following single doses of aspirin. Arthritis and rheumatism. 1986;29(8):971–980. - PubMed
-
- Savides MC, Oehme FW, Nash SL, et al. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol Appl Pharmacol. 1984;74(1):26–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

