Elimination of Aspergillus fumigatus conidia from the airways of mice with allergic airway inflammation
- PMID: 23890251
- PMCID: PMC3735401
- DOI: 10.1186/1465-9921-14-78
Elimination of Aspergillus fumigatus conidia from the airways of mice with allergic airway inflammation
Abstract
Background: Aspergillus fumigatus conidia can exacerbate asthma symptoms. Phagocytosis of conidia is a principal component of the host antifungal defense. We investigated whether allergic airway inflammation (AAI) affects the ability of phagocytic cells in the airways to internalize the resting fungal spores.
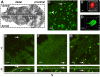
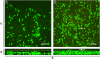
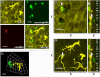
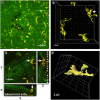
Methods: Using BALB/c mice with experimentally induced AAI, we tested the ability of neutrophils, macrophages, and dendritic cells to internalize A. fumigatus conidia at various anatomical locations. We used light microscopy and differential cell and conidium counts to determine the ingestion potential of neutrophils and macrophages present in bronchoalveolar lavage (BAL). To identify phagocyte-conidia interactions in conducting airways, conidia labeled with tetramethylrhodamine-(5-(and-6))-isothiocyanate were administered to the oropharyngeal cavity of mice. Confocal microscopy was used to quantify the ingestion potential of Ly-6G+ neutrophils and MHC II+ antigen-presenting cells located in the intraepithelial and subepithelial areas of conducting airways.
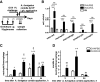
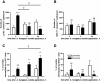
Results: Allergen challenge induced transient neutrophil recruitment to the airways. Application of A. fumigatus conidia at the acute phase of AAI provoked recurrent neutrophil infiltration, and consequently increased the number and the ingestion potential of the airway neutrophils. In the absence of recurrent allergen or conidia provocation, both the ingestion potential and the number of BAL neutrophils decreased. As a result, conidia were primarily internalized by alveolar macrophages in both AAI and control mice at 24 hours post-inhalation. Transient influx of neutrophils to conducting airways shortly after conidial application was observed in mice with AAI. In addition, the ingestion potential of conducting airway neutrophils in mice with induced asthma exceeded that of control mice. Although the number of neutrophils subsequently decreased, the ingestion capacity remained elevated in AAI mice, even at 24 hours post-conidia application.
Conclusions: Aspiration of allergen to sensitized mice enhanced the ingestion potential of conducting airway neutrophils. Such activation primes neutrophils so that they are sufficient to control dissemination of non-germinating A. fumigatus conidia. At the same time, it can be a reason for the development of sensitivity to fungi and subsequent asthma exacerbation.
Figures
Similar articles
-
Murine Intraepithelial Dendritic Cells Interact With Phagocytic Cells During Aspergillus fumigatus-Induced Inflammation.Front Immunol. 2020 Feb 25;11:298. doi: 10.3389/fimmu.2020.00298. eCollection 2020. Front Immunol. 2020. PMID: 32161590 Free PMC article.
-
An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice.Med Mycol. 2010 Dec;48(8):1056-65. doi: 10.3109/13693786.2010.485582. Epub 2010 May 20. Med Mycol. 2010. PMID: 20482452 Free PMC article.
-
Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia.J Immunol. 2001 Feb 1;166(3):1832-42. doi: 10.4049/jimmunol.166.3.1832. J Immunol. 2001. PMID: 11160230
-
Interaction of phagocytes with filamentous fungi.Curr Opin Microbiol. 2010 Aug;13(4):409-15. doi: 10.1016/j.mib.2010.04.009. Epub 2010 Jun 2. Curr Opin Microbiol. 2010. PMID: 20627805 Review.
-
Phagocyte responses towards Aspergillus fumigatus.Int J Med Microbiol. 2011 Jun;301(5):436-44. doi: 10.1016/j.ijmm.2011.04.012. Epub 2011 May 14. Int J Med Microbiol. 2011. PMID: 21571589 Review.
Cited by
-
Murine Intraepithelial Dendritic Cells Interact With Phagocytic Cells During Aspergillus fumigatus-Induced Inflammation.Front Immunol. 2020 Feb 25;11:298. doi: 10.3389/fimmu.2020.00298. eCollection 2020. Front Immunol. 2020. PMID: 32161590 Free PMC article.
-
Impact of CD11c+ cells in conducting airway lumen on Aspergillus fumigatus conidia deposition in neutropenic mice.Front Fungal Biol. 2025 Jun 18;6:1591891. doi: 10.3389/ffunb.2025.1591891. eCollection 2025. Front Fungal Biol. 2025. PMID: 40606830 Free PMC article.
-
Mouse pneumonia model by Acinetobacter baumannii multidrug resistant strains: Comparison between intranasal inoculation, intratracheal instillation and oropharyngeal aspiration techniques.PLoS One. 2021 Dec 2;16(12):e0260627. doi: 10.1371/journal.pone.0260627. eCollection 2021. PLoS One. 2021. PMID: 34855837 Free PMC article.
-
Oropharyngeal aspiration of Burkholderia mallei and Burkholderia pseudomallei in BALB/c mice.PLoS One. 2014 Dec 11;9(12):e115066. doi: 10.1371/journal.pone.0115066. eCollection 2014. PLoS One. 2014. PMID: 25503969 Free PMC article.
-
Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy.J Fungi (Basel). 2021 Jun 7;7(6):454. doi: 10.3390/jof7060454. J Fungi (Basel). 2021. PMID: 34200399 Free PMC article.
References
-
- Fukushima C, Matsuse H, Fukahori S, Tsuchida T, Kawano T, Senjyu H, Kohno S. Aspergillus fumigatus synergistically enhances mite-induced allergic airway inflammation. Med Sci Monit. 2010;16(7):BR197–202. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

