Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial
- PMID: 23890405
- PMCID: PMC3729430
- DOI: 10.1186/1471-2334-13-348
Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial
Abstract
Background: Improved influenza vaccines are needed to reduce influenza-associated complications in older adults. The aim of this study was to identify the optimal formulation of adjuvanted seasonal influenza vaccine for use in elderly people.
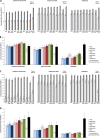
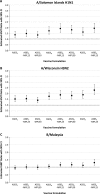
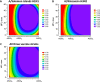
Methods: This observer-blind, randomized study assessed the optimal formulation of adjuvanted seasonal influenza vaccine based on immunogenicity and safety in participants aged ≥65 years. Participants were randomized (~200 per group) to receive one dose of non-adjuvanted vaccine or one of eight formulations of vaccine formulated with a squalene and tocopherol oil-in-water emulsion-based Adjuvant System (AS03(C), AS03(B) or AS03(A), with 2.97, 5.93 and 11.86 mg tocopherol, respectively) together with the immunostimulant monophosphoryl lipid A (MPL, doses of 0, 25 or 50 mg). Hemagglutination-inhibition (HI) antibody responses and T-cell responses were assessed on Day 0 and 21 days post-vaccination. The ratio of HI-based geometric mean titers in adjuvanted versus non-adjuvanted vaccine groups were calculated and the lower limit of the 90% confidence interval was transformed into a desirability index (a value between 0 and 1) in an experimental domain for each vaccine strain, and plotted in relation to the AS03 and MPL dose combination in the formulation. This model was used to assess the optimal formulation based on HI antibody titers. Reactogenicity and safety were also assessed. The immunogenicity and safety analyses were used to evaluate the optimal formulation of adjuvanted vaccine.
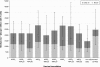
Results: In the HI antibody-based model, an AS03 dose-response was evident; responses against the A/H1N1 and A/H3N2 strains were higher for all adjuvanted formulations versus non-adjuvanted vaccine, and for the AS03(A)-MPL25, AS03(B)-MPL25 and AS03(B)-MPL50 formulations against the B strain. Modelling using more stringent criteria (post hoc) showed a clear dose-range effect for the AS03 component against all strains, whereas MPL showed a limited effect. Higher T-cell responses for adjuvanted versus non-adjuvanted vaccine were observed for all except two formulations (AS03(C) and AS03(B)-MPL25). Reactogenicity increased with increasing AS03 dosage, and with MPL. No safety concerns were raised.
Conclusions: Five formulations containing AS03(A) or AS03(B) were identified as potential candidates to improve immune responses to influenza vaccination; AS03(B) without MPL showed the best balance between improved immunogenicity and acceptable reactogenicity.
Trial registration: This trial is registered at ClinicalTrials.gov, NCT00540592.
Figures


References
-
- Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172(6):3437–3446. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

