If we know so much about preeclampsia, why haven't we cured the disease?
- PMID: 23890710
- PMCID: PMC4066309
- DOI: 10.1016/j.jri.2013.05.003
If we know so much about preeclampsia, why haven't we cured the disease?
Abstract
Preeclampsia has been recognized for at least 100 years. In the last 20 years, the consideration of the disorder as more than simply hypertension in pregnancy has led to an explosion in knowledge about preeclampsia pathophysiology. It is now evident that for most cases of preeclampsia, the root cause is the placenta. Relatively reduced placental perfusion leads to inflammation, oxidative stress, and endoplasmic reticulum stress, which converge to modify maternal physiology, with endothelium an important target. Although preeclampsia is characteristically diagnosed in the last third of pregnancy, it is evident that many of these pathophysiological changes can be detected long before clinically evident disease. Furthermore, it is evident that the "maternal constitution," including genetic, behavioral, and metabolic factors, influences the maternal response to the abnormal placentation of preeclampsia. These insights would seem to provide a guide for the prediction of the disorder in early pregnancy, along with targets for intervention. However, this has not been the case. Predictive tests guided by this knowledge do not predict well and several interventions guided by the expanded understanding of pathophysiology do not prevent the disease. We propose that these failures are secondary to the fact that preeclampsia is more than one disorder. Further, we suggest that future progress toward prediction and prevention will require research guided by this concept.
Keywords: Pathophysiology; Prediction; Preeclampsia; Prevention; Translation.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures


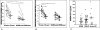
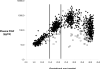
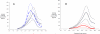
References
-
- AARDEMA MW, OOSTERHOF H, TIMMER A, VAN ROOY I, AARNOUDSE JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22:405–1. - PubMed
-
- ASKIE LM, DULEY L, HENDERSON-SMART DJ, STEWART LA, GROUP PC. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–8. - PubMed
-
- BLASCHITZ A, HUTTER H, DOHR G. HLA Class I protein expression in the human placenta. Early Pregnancy [Electronic Resource] 2001;5:67–9. - PubMed
-
- BORZYCHOWSKI AM, SARGENT IL, REDMAN CWG. Inflammation and pre-eclampsia. Seminars In Fetal & Neonatal Medicine. 2006;11:309–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

