Progression from ductal carcinoma in situ to invasive breast cancer: revisited
- PMID: 23890733
- PMCID: PMC5528459
- DOI: 10.1016/j.molonc.2013.07.005
Progression from ductal carcinoma in situ to invasive breast cancer: revisited
Abstract
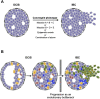
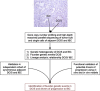
Ductal carcinoma in situ (DCIS) is an intraductal neoplastic proliferation of epithelial cells that is separated from the breast stroma by an intact layer of basement membrane and myoepithelial cells. DCIS is a non-obligate precursor of invasive breast cancer, and up to 40% of these lesions progress to invasive disease if untreated. Currently, it is not possible to predict accurately which DCIS would be more likely to progress to invasive breast cancer as neither the significant drivers of the invasive transition have been identified, nor has the clinical utility of tests predicting the likelihood of progression been demonstrated. Although molecular studies have shown that qualitatively, synchronous DCIS and invasive breast cancers are remarkably similar, there is burgeoning evidence to demonstrate that intra-tumor genetic heterogeneity is observed in a subset of DCIS, and that the process of progression to invasive disease may constitute an 'evolutionary bottleneck', resulting in the selection of subsets of tumor cells with specific genetic and/or epigenetic aberrations. Here we review the clinical challenge posed by DCIS, the contribution of the microenvironment and genetic aberrations to the progression from in situ to invasive breast cancer, the emerging evidence of the impact of intra-tumor genetic heterogeneity on this process, and strategies to combat this heterogeneity.
Keywords: Breast cancer; Darwinian evolution; Genomics; Intra-tumor genetic heterogeneity; Intraductal.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Abdel-Fatah, T.M. , Powe, D.G. , Hodi, Z. , Lee, A.H. , Reis-Filho, J.S. , Ellis, I.O. , 2007. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. The American Journal of Surgical Pathology. 31, 417–426. - PubMed
-
- Allinen, M. , Beroukhim, R. , Cai, L. , Brennan, C. , Lahti-Domenici, J. , Huang, H. , Porter, D. , Hu, M. , Chin, L. , Richardson, A. , Schnitt, S. , Sellers, W.R. , Polyak, K. , 2004. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 6, 17–32. - PubMed
-
- American Cancer Society, 2013. Cancer Facts and Figures 2013 American Cancer Society; Atlanta, GA:
-
- Aubele, M. , Mattis, A. , Zitzelsberger, H. , Walch, A. , Kremer, M. , Hutzler, P. , Hofler, H. , Werner, M. , 1999. Intratumoral heterogeneity in breast carcinoma revealed by laser-microdissection and comparative genomic hybridization. Cancer Genetics and Cytogenetics. 110, 94–102. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

