Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis
- PMID: 23890799
- PMCID: PMC9316415
- DOI: 10.1016/j.mehy.2013.06.026
Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis
Abstract
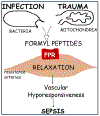
Sepsis is a major cause of mortality and morbidity in trauma patients despite aggressive treatment. Traumatic injury may trigger infective or non-infective systemic inflammatory response syndrome (SIRS) and sepsis. Sepsis and SIRS are accompanied by an inability to regulate the inflammatory response but the cause of this perturbation is still unknown. The major pathophysiological characteristic of sepsis is the vascular collapse (i.e., loss of control of vascular tone); however, at the cellular level the final mediator of extreme vasodilatation has yet to be identified. After trauma, cellular injury releases endogenous damage-associated molecular patterns (DAMPs) that activate the innate immune system. Mitochondrial DAMPs express at least two molecular signatures, N-formyl peptides and mitochondrial DNA that act on formyl peptide receptors (FPRs) and Toll-like receptor 9, respectively. N-Formyl peptides are potent immunocyte activators and, once released in the circulation, they induce modulation of vascular tone by cellular mechanisms that are not completely understood. We have observed that N-formyl peptides from bacterial (FMLP) and mitochondrial (FMIT) sources induce FPR-mediated vasodilatation in resistance arteries. Accordingly, we propose that tissue and cellular trauma induces the release of N-formyl peptides from mitochondria triggering inflammation and vascular collapse via activation of FPR and contributing to the development of sepsis. The proposed hypothesis provides clinically significant information linking trauma, mitochondrial N-formyl peptides and inflammation to vascular collapse and sepsis. If our hypothesis is true, it may lead to new strategies in the management of sepsis that can help clinicians effectively manage non-infectious and infectious inflammatory responses.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
None.
Figures

References
-
- WHO. Global forum on trauma care. World Health Organization; 2009.
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. - PubMed
-
- Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med 2003;9:517–24. - PubMed
-
- Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med 2001;29(Suppl.):S109–16. - PubMed
-
- Cohen J The immunopsthogenesis os sepsis. Nature 2002;420:885–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

