Vascular priming enhances chemotherapeutic efficacy against head and neck cancer
- PMID: 23890930
- PMCID: PMC3772633
- DOI: 10.1016/j.oraloncology.2013.06.011
Vascular priming enhances chemotherapeutic efficacy against head and neck cancer
Abstract
Purpose: The need to improve chemotherapeutic efficacy against head and neck squamous cell carcinomas (HNSCC) is well recognized. In this study, we investigated the potential of targeting the established tumor vasculature in combination with chemotherapy in head and neck cancer.
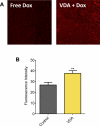
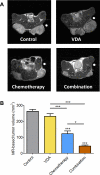
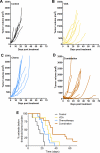
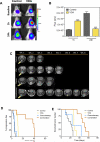
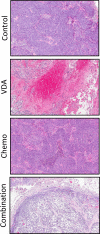
Methods: Experimental studies were carried out in multiple human HNSCC xenograft models to examine the activity of the vascular disrupting agent (VDA) 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in combination with chemotherapy. Multimodality imaging (magnetic resonance imaging, bioluminescence) in conjunction with drug delivery assessment (fluorescence microscopy), histopathology and microarray analysis was performed to characterize tumor response to therapy. Long-term treatment outcome was assessed using clinically-relevant end points of efficacy.
Results: Pretreatment of tumors with VDA prior to administration of chemotherapy increased intratumoral drug delivery and treatment efficacy. Enhancement of therapeutic efficacy was dependent on the dose and duration of VDA treatment but was independent of the chemotherapeutic agent evaluated. Combination treatment resulted in increased tumor cell kill and improvement in progression-free survival and overall survival in both ectopic and orthotopic HNSCC models.
Conclusion: Our results show that preconditioning of the tumor microenvironment with an antivascular agent primes the tumor vasculature and results in enhancement of chemotherapeutic delivery and efficacy in vivo. Further investigation into the activity of antivascular agents in combination with chemotherapy against HNSCC is warranted.
Keywords: Angiogenesis; Head and neck squamous cell carcinoma; Vascular disrupting agents; Vascular targeting.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts.Neoplasia. 2006 Jul;8(7):534-42. doi: 10.1593/neo.06295. Neoplasia. 2006. PMID: 16867215 Free PMC article.
-
Monitoring antivascular therapy in head and neck cancer xenografts using contrast-enhanced MR and US imaging.Angiogenesis. 2011 Dec;14(4):491-501. doi: 10.1007/s10456-011-9233-1. Epub 2011 Sep 7. Angiogenesis. 2011. PMID: 21901534 Free PMC article.
-
Bevacizumab enhances the therapeutic efficacy of Irinotecan against human head and neck squamous cell carcinoma xenografts.Oral Oncol. 2011 Jun;47(6):459-66. doi: 10.1016/j.oraloncology.2011.04.001. Epub 2011 May 6. Oral Oncol. 2011. PMID: 21530364 Free PMC article.
-
Disrupting established tumor blood vessels: an emerging therapeutic strategy for cancer.Cancer. 2010 Apr 15;116(8):1859-71. doi: 10.1002/cncr.24975. Cancer. 2010. PMID: 20166210 Review.
-
5,6-dimethylxanthenone-4-acetic acid (DMXAA): a new biological response modifier for cancer therapy.Invest New Drugs. 2002 Aug;20(3):281-95. doi: 10.1023/a:1016215015530. Invest New Drugs. 2002. PMID: 12201491 Review.
Cited by
-
ASA404, a vascular disrupting agent, as an experimental treatment approach for brain tumors.Oncol Lett. 2017 Nov;14(5):5443-5451. doi: 10.3892/ol.2017.6832. Epub 2017 Aug 28. Oncol Lett. 2017. PMID: 29098034 Free PMC article.
-
LCRF-0006, a small molecule mimetic of the N-cadherin antagonist peptide ADH-1, synergistically increases multiple myeloma response to bortezomib.FASEB Bioadv. 2020 Jun 15;2(6):339-353. doi: 10.1096/fba.2019-00073. eCollection 2020 Jun. FASEB Bioadv. 2020. PMID: 32617520 Free PMC article.
-
Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors.Molecules. 2021 Apr 27;26(9):2551. doi: 10.3390/molecules26092551. Molecules. 2021. PMID: 33925707 Free PMC article. Review.
-
Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer.Cancers (Basel). 2016 Jan 7;8(1):11. doi: 10.3390/cancers8010011. Cancers (Basel). 2016. PMID: 26751478 Free PMC article.
-
Synchronous Intravital Imaging and Cavitation Monitoring of Antivascular Focused Ultrasound in Tumor Microvasculature Using Monodisperse Low Boiling Point Nanodroplets.ACS Nano. 2024 Jan 9;18(1):410-427. doi: 10.1021/acsnano.3c07711. Epub 2023 Dec 26. ACS Nano. 2024. PMID: 38147452 Free PMC article.
References
-
- Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55:242–8. - PubMed
-
- Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100:33–40. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285:1182–6. - PubMed
-
- Zätterström UK, Brun E, Willén R, Kjellén E, Wennerberg J. Tumor angiogenesis and prognosis in squamous cell carcinoma of the head and neck. Head Neck. 1995;17:312–8. - PubMed
-
- Galmarini FC, Galmarini CM, Sarchi MI, Abulafia J, Galmirini D. Heterogeneous distribution of tumor blood supply affects the response to chemotherapy in patients with head and neck cancer. Microcirculation. 2000;7:405–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

