Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia
- PMID: 23891112
- PMCID: PMC3770898
- DOI: 10.1016/j.cub.2013.07.017
Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia
Abstract
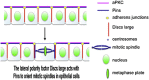
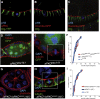
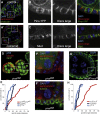
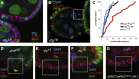
Mitotic spindles in epithelial cells are oriented in the plane of the epithelium so that both daughter cells remain within the monolayer, and defects in spindle orientation have been proposed to promote tumorigenesis by causing epithelial disorganization and hyperplasia. Previous work has implicated the apical polarity factor aPKC, the junctional protein APC2, and basal integrins in epithelial spindle orientation, but the underlying mechanisms remain unclear. We show that these factors are not required for spindle orientation in the Drosophila follicular epithelium. Furthermore, aPKC and other apical polarity factors disappear from the apical membrane in mitosis. Instead, spindle orientation requires the lateral factor Discs large (Dlg), a function that is separable from its role in epithelial polarity. In neuroblasts, Pins recruits Dlg and Mud to form an apical complex that orients spindles along the apical-basal axis. We show that Pins and Mud are also necessary for spindle orientation in follicle cells, as is the interaction between Dlg and Pins. Dlg localizes independently of Pins, however, suggesting that its lateral localization determines the planar orientation of the spindle in epithelial cells. Thus, different mechanisms recruit the conserved Dlg/Pins/Mud complex to orient the spindle in opposite directions in distinct cell types.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- McCaffrey L.M., Macara I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727–735. - PubMed
-
- Fernández-Miñán A., Martín-Bermudo M.D., González-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 2007;17:683–688. - PubMed
-
- Lu B., Roegiers F., Jan L.Y., Jan Y.N. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. - PubMed
-
- McCartney B.M., Price M.H., Webb R.L., Hayden M.A., Holot L.M., Zhou M., Bejsovec A., Peifer M. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 2006;133:2407–2418. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

