Peroxiredoxin-1 from the human hookworm Ancylostoma ceylanicum forms a stable oxidized decamer and is covalently inhibited by conoidin A
- PMID: 23891152
- PMCID: PMC3755041
- DOI: 10.1016/j.chembiol.2013.06.011
Peroxiredoxin-1 from the human hookworm Ancylostoma ceylanicum forms a stable oxidized decamer and is covalently inhibited by conoidin A
Abstract
Hookworms are parasitic nematodes that have a devastating impact on global health, particularly in developing countries. We report a biochemical and structural analysis of a peroxiredoxin from the hookworm Ancylostoma ceylanicum, AcePrx-1. Peroxiredoxins provide antioxidant protection and act as signaling molecules and chaperones. AcePrx-1 is expressed in adult hookworms and can be inactivated by 2,3-bis(bromomethyl)quinoxaline-1,4-dioxide (conoidin A). Conoidin A inactivates AcePrx-1 by alkylating or crosslinking the catalytic cysteines, while maintaining the enzyme in the "locally unfolded" conformation. Irreversible oxidation of the resolving cysteine may contribute additional inhibitory activity. A crystal structure of oxidized AcePrx-1 reveals a disulfide-linked decamer. A helix macrodipole near the active site increases the reactivity of the catalytic cysteines to conoidin A. This work demonstrates the promise of conoidin compounds as probes to evaluate peroxiredoxins as drug targets in human parasites.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

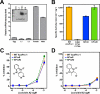


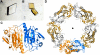

References
-
- Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. - PubMed
-
- Bundy D, Sher A, Michael E. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:273–274. - PubMed
-
- Bungiro R, Cappello M. Twenty-first century progress toward the global control of human hookworm infection. Curr Infect Dis Rep. 2011;13:210–217. - PubMed
-
- Cao Z, Bhella D, Lindsay JG. Reconstitution of the mitochondrial PrxIII antioxidant defence pathway: general properties and factors affecting PrxIII activity and oligomeric state. J. Mol. Biol. 2007;372:1022–1033. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

