Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines
- PMID: 23891185
- PMCID: PMC3947850
- DOI: 10.1016/j.bmcl.2013.06.048
Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines
Abstract
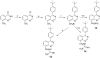
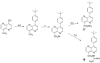
Several phenyl substituted naphthalenes and isoquinolines have been identified as antibacterial agents that inhibit FtsZ-Zing formation. In the present study we evaluated the antibacterial of several phenyl substituted quinoxalines, quinazolines and 1,5-naphthyridines against methicillin-sensitive and methicillin-resistant Staphylococcusaureus and vancomycin-sensitive and vancomycin-resistant Enterococcusfaecalis. Some of the more active compounds against S. aureus were evaluated for their effect on FtsZ protein polymerization. Further studies were also performed to assess their relative bactericidal and bacteriostatic activities. The notable differences observed between nonquaternized and quaternized quinoxaline derivatives suggest that differing mechanisms of action are associated with their antibacterial properties.
Keywords: 1,5-Naphthyridines; Antibiotics; Bactericidal; Bacteriostatic; Enterococcus faecalis; Quinazolines; Quinoxalines; Staphylococcus aureus.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



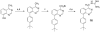
References
-
- Arias CA, Murray BE. N. Eng.J. Med. 2009;360
-
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. Lancet Infect. Dis. 2010;10:597. - PMC - PubMed
-
- Livermore DM. J. Antimicrob. Chemother. 2009;64(Suppl. 1):i29. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. J. Am. Med. Assoc. 2007;298:1763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

