Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia
- PMID: 23891471
- PMCID: PMC3738835
- DOI: 10.1016/j.ajhg.2013.07.009
Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia
Abstract
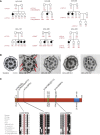
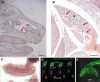
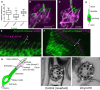
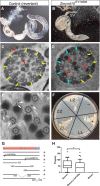
Primary ciliary dyskinesia (PCD) is a ciliopathy characterized by airway disease, infertility, and laterality defects, often caused by dual loss of the inner dynein arms (IDAs) and outer dynein arms (ODAs), which power cilia and flagella beating. Using whole-exome and candidate-gene Sanger resequencing in PCD-affected families afflicted with combined IDA and ODA defects, we found that 6/38 (16%) carried biallelic mutations in the conserved zinc-finger gene BLU (ZMYND10). ZMYND10 mutations conferred dynein-arm loss seen at the ultrastructural and immunofluorescence level and complete cilia immotility, except in hypomorphic p.Val16Gly (c.47T>G) homozygote individuals, whose cilia retained a stiff and slowed beat. In mice, Zmynd10 mRNA is restricted to regions containing motile cilia. In a Drosophila model of PCD, Zmynd10 is exclusively expressed in cells with motile cilia: chordotonal sensory neurons and sperm. In these cells, P-element-mediated gene silencing caused IDA and ODA defects, proprioception deficits, and sterility due to immotile sperm. Drosophila Zmynd10 with an equivalent c.47T>G (p.Val16Gly) missense change rescued mutant male sterility less than the wild-type did. Tagged Drosophila ZMYND10 is localized primarily to the cytoplasm, and human ZMYND10 interacts with LRRC6, another cytoplasmically localized protein altered in PCD. Using a fly model of PCD, we conclude that ZMYND10 is a cytoplasmic protein required for IDA and ODA assembly and that its variants cause ciliary dysmotility and PCD with laterality defects.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. - PubMed
-
- Broadhead R., Dawe H.R., Farr H., Griffiths S., Hart S.R., Portman N., Shaw M.K., Ginger M.L., Gaskell S.J., McKean P.G., Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. - PubMed
-
- Lindemann C.B., Lesich K.A. Flagellar and ciliary beating: the proven and the possible. J. Cell Sci. 2010;123:519–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

