Current development in isoprenoid precursor biosynthesis and regulation
- PMID: 23891475
- PMCID: PMC4068245
- DOI: 10.1016/j.cbpa.2013.06.020
Current development in isoprenoid precursor biosynthesis and regulation
Abstract
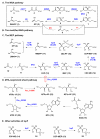
Isoprenoids are one of the largest classes of natural products and all of them are constructed from two precursors, isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). For decades, the mevalonic acid (MVA) pathway was proposed to be the only IPP and DMAPP biosynthetic pathway. This review summarizes the newly discovered IPP and DMAPP production pathways since late 1990s, their distribution among different kingdoms, and their roles in secondary metabolite production. These new IPP and DMAPP production pathways include the methylerythritol phosphate (MEP) pathway, a modified MVA pathway, and the 5-methylthioadenosine shunt pathway. Relative to the studies on the MVA pathway, information on the MEP pathway regulation is limited and the mechanistic details of several of its novel transformations remain to be addressed. Current status on both MEP pathway regulation and mechanistic issues is also presented.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
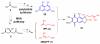



References
-
- Breitmaier E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. WILEY-VCH, Weinheim; Germany: 2006. p. ix.
-
- Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW. Biosynthesis of mevalonic acid from acetyl-CoA. In: Cane DE, editor. Comprehensive Natural Product Chemistry. Pergamon; Oxford: 1999. pp. 15–44.
-
- Agranoff BW, Eggerer H, Henning U, Lynen F. Isopentenyl pyrophosphate isomerase. J Am Chem Soc. 1959;81:1254–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

