Review: Glycation of human serum albumin
- PMID: 23891854
- PMCID: PMC3795802
- DOI: 10.1016/j.cca.2013.07.013
Review: Glycation of human serum albumin
Abstract
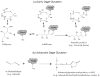
Glycation involves the non-enzymatic addition of reducing sugars and/or their reactive degradation products to amine groups on proteins. This process is promoted by the presence of elevated blood glucose concentrations in diabetes and occurs with various proteins that include human serum albumin (HSA). This review examines work that has been conducted in the study and analysis of glycated HSA. The general structure and properties of HSA are discussed, along with the reactions that can lead to modification of this protein during glycation. The use of glycated HSA as a short-to-intermediate term marker for glycemic control in diabetes is examined, and approaches that have been utilized for measuring glycated HSA are summarized. Structural studies of glycated HSA are reviewed, as acquired for both in vivo and in vitro glycated HSA, along with data that have been obtained on the rate and thermodynamics of HSA glycation. In addition, this review considers various studies that have investigated the effects of glycation on the binding of HSA with drugs, fatty acids and other solutes and the potential clinical significance of these effects.
Keywords: Diabetes; Drug–protein binding; Glycated albumin; Glycation; Human serum albumin; Protein modification.
© 2013.
Figures
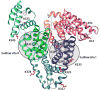
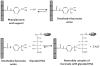
References
-
- International Diabetes Federation diabetes atlas. 5. Brussels, Belgium: International Diabetes Federation; 2011.
-
- National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2011. U.S. Centers for Disease Control; Atlanta, GA: 2011.
-
- Nelson DL, Cox MM, editors. Lehninger principles of biochemistry. New York: W. H. Freeman and Company; 2005.
-
- Hartog JWL, Voors AA, Bakker SJL, Smit AJ, Veldhuisen DJV. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–55. - PubMed
-
- Olijhoeka JK, Graafb YVD, Bangaa JD, Algrab A, Rabelinka TJ, Visserena FLJ. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur Heart J. 2004;25:342–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

