Species sequence differences determine the interaction of GnRH receptor with the cellular quality control system
- PMID: 23891857
- PMCID: PMC3795929
- DOI: 10.1016/j.mce.2013.07.012
Species sequence differences determine the interaction of GnRH receptor with the cellular quality control system
Abstract
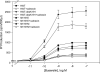
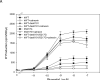
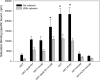
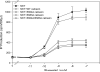
Plasma membrane expression (PME) of the human GnRHR (hGnRHR) is regulated by a primate-specific Lys(191) which destabilizes a Cys(14)-Cys(200) bridge required by the cellular quality control system (QCS). A 4-amino, non-contiguous "motif" (Leu(112), Gln(208), Leu(300), Asp(302)) is required for this effect. The hGnRHR sequence, with or without Lys(191), decreases PME and inositol phosphate (IP) production when co-expressed with calnexin, a QCS chaperone. WT rat GnRHR, decreases PME and IP production, when co-expressed with calnexin, but to a lesser degree than hGnRH. When the human sequence contains the rat motif, IP production is closer to that of rat GnRHR. When Lys(191) is deleted from hGnRHR and co-expressed with calnexin, IP production is similar to the rat sequence. When rat GnRHR containing Lys(191) and the human motif is co-expressed with calnexin, IP production is similar to cells expressing the hGnRHR. The motif sequence appears to be a determinant of calnexin recognition.
Keywords: Calnexin; ER protein retention; GPCR; GnRH receptor; Protein trafficking.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures




Similar articles
-
Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy.J Biol Chem. 2006 Mar 31;281(13):8417-25. doi: 10.1074/jbc.M510601200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446355
-
Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression.J Mol Endocrinol. 2006 Dec;37(3):479-88. doi: 10.1677/jme.1.02142. J Mol Endocrinol. 2006. PMID: 17170088
-
Protein disulfide isomerase chaperone ERP-57 decreases plasma membrane expression of the human GnRH receptor.Cell Biochem Funct. 2010 Jan;28(1):66-73. doi: 10.1002/cbf.1622. Cell Biochem Funct. 2010. PMID: 20029959 Free PMC article.
-
Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans.J Clin Endocrinol Metab. 2003 Jul;88(7):3360-7. doi: 10.1210/jc.2003-030084. J Clin Endocrinol Metab. 2003. PMID: 12843188
-
Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease.Mol Cell Endocrinol. 2009 Feb 27;299(2):137-45. doi: 10.1016/j.mce.2008.10.051. Epub 2008 Nov 18. Mol Cell Endocrinol. 2009. PMID: 19059461 Free PMC article. Review.
Cited by
-
Chaperoning G protein-coupled receptors: from cell biology to therapeutics.Endocr Rev. 2014 Aug;35(4):602-47. doi: 10.1210/er.2013-1121. Epub 2014 Mar 24. Endocr Rev. 2014. PMID: 24661201 Free PMC article. Review.
References
-
- Brothers SP, et al. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–97. - PubMed
-
- Brothers SP, et al. Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2003;88:6107–12. - PubMed
-
- Brothers SP, et al. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–88. - PubMed
-
- Brothers SP, et al. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol. 2002;190:19–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

