Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model
- PMID: 23891888
- PMCID: PMC4109283
- DOI: 10.1016/j.expneurol.2013.07.010
Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model
Abstract
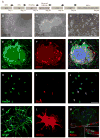
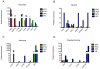
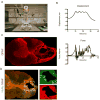
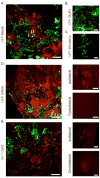
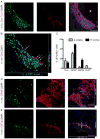
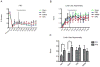
Neural progenitor cells (NPCs) have shown modest potential and some side effects (e.g. allodynia) for treatment of spinal cord injury (SCI). In only a few cases, however, have NPCs shown promise at the chronic stage. Given the 1.275 million people living with chronic paralysis, there is a significant need to rigorously evaluate the cell types and methods for safe and efficacious treatment of this devastating condition. For the first time, we examined the pre-clinical potential of NPCs derived from human induced pluripotent stem cells (hiPSCs) to repair chronic SCI. hiPSCs were differentiated into region-specific (i.e. caudal) NPCs, then transplanted into a new, clinically relevant model of early chronic cervical SCI. We established the conditions for successful transplantation of caudalized hiPSC-NPCs and demonstrate their remarkable ability to integrate and produce multiple neural lineages in the early chronic injury environment. In contrast to prior reports in acute and sub-acute injury models, survival and integration of hiPSC-derived neural cells in the early chronic cervical model did not lead to significant improvement in forelimb function or induce allodynia. These data indicate that while hiPSCs show promise, future work needs to focus on the specific hiPSC-derivatives or co-therapies that will restore function in the early chronic injury setting.
Keywords: Induced pluripotent stem cells; Neural progenitor cells; Spinal cord injury.
© 2013.
Figures

Similar articles
-
Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity.PLoS One. 2012;7(12):e52787. doi: 10.1371/journal.pone.0052787. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300777 Free PMC article.
-
Human neural progenitors derived from integration-free iPSCs for SCI therapy.Stem Cell Res. 2017 Mar;19:55-64. doi: 10.1016/j.scr.2017.01.004. Epub 2017 Jan 5. Stem Cell Res. 2017. PMID: 28073086 Free PMC article.
-
Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury.J Neurotrauma. 2007 May;24(5):835-45. doi: 10.1089/neu.2006.3771. J Neurotrauma. 2007. PMID: 17518538
-
Human stem cell-derived neurons and neural circuitry therapeutics: Next frontier in spinal cord injury repair.Exp Biol Med (Maywood). 2022 Dec;247(23):2142-2151. doi: 10.1177/15353702221114099. Epub 2022 Aug 16. Exp Biol Med (Maywood). 2022. PMID: 35974701 Free PMC article. Review.
-
A Review of Treatment Methods Focusing on Human Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cell Transplantation for Chronic Spinal Cord Injury.Medicina (Kaunas). 2023 Jul 1;59(7):1235. doi: 10.3390/medicina59071235. Medicina (Kaunas). 2023. PMID: 37512047 Free PMC article. Review.
Cited by
-
Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury.Exp Neurol. 2015 Sep;271:479-92. doi: 10.1016/j.expneurol.2015.07.020. Epub 2015 Jul 26. Exp Neurol. 2015. PMID: 26216662 Free PMC article.
-
The combination of forskolin and VPA increases gene expression efficiency to the hypoxia/neuron-specific system.Ann Transl Med. 2020 Aug;8(15):933. doi: 10.21037/atm-20-3871. Ann Transl Med. 2020. PMID: 32953733 Free PMC article.
-
The Effect of iPS-Derived Neural Progenitors Seeded on Laminin-Coated pHEMA-MOETACl Hydrogel with Dual Porosity in a Rat Model of Chronic Spinal Cord Injury.Cell Transplant. 2019 Apr;28(4):400-412. doi: 10.1177/0963689718823705. Epub 2019 Jan 18. Cell Transplant. 2019. PMID: 30654639 Free PMC article.
-
Therapeutic intraspinal stimulation to generate activity and promote long-term recovery.Front Neurosci. 2014 Feb 27;8:21. doi: 10.3389/fnins.2014.00021. eCollection 2014. Front Neurosci. 2014. PMID: 24578680 Free PMC article.
-
Stem Cells Therapy for Spinal Cord Injury.Int J Mol Sci. 2018 Mar 30;19(4):1039. doi: 10.3390/ijms19041039. Int J Mol Sci. 2018. PMID: 29601528 Free PMC article. Review.
References
-
- Prevalence of Paralysis. 2009 Retrieved May 17, 2012, from http://www.christopherreeve.org/site/c.mtKZKgMWKwG/b.5184255/k.6D74/Prev....
-
- Spinal Cord Injury Facts and Figures at a Glance. Retrieved May 17, 2012, from https://www.nscisc.uab.edu/PublicDocuments/nscisc_home/pdf/Facts%202011%....
-
- Adachi K, Suemori H, et al. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15(5):455–470. - PubMed
-
- Alaverdashvili M, Whishaw IQ. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010;167(1):21–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

