Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase
- PMID: 23891899
- PMCID: PMC4870004
- DOI: 10.1016/j.pain.2013.07.032
Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase
Abstract
Many of the widely used anticancer drugs induce dose-limiting peripheral neuropathies that undermine their therapeutic efficacy. Animal models of chemotherapy-induced painful peripheral neuropathy (CIPN) evoked by a variety of drug classes, including taxanes, vinca alkaloids, platinum-complexes, and proteasome-inhibitors, suggest that the common underlying mechanism in the development of these neuropathies is mitotoxicity in primary nerve sensory axons (PNSAs) arising from reduced mitochondrial bioenergetics [eg adenosine triphosphate (ATP) production deficits due to compromised respiratory complex I and II activity]. The causative mechanisms of this mitotoxicity remain poorly defined. However, peroxynitrite, an important pro-nociceptive agent, has been linked to mitotoxicity in several disease states and may also drive the mitotoxicity associated with CIPN. Our findings reveal that the development of mechano-hypersensitivity induced by paclitaxel, oxaliplatin, and bortezomib was prevented by administration of the peroxynitrite decomposition catalyst Mn(III) 5,10,15,20-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin (MnTE-2-PyP(5+)) without interfering with their anti-tumor effects. Peak CIPN was associated with the nitration and inactivation of superoxide dismutase in the mitochondria, but not in the cytosol, as well as a significant decrease in ATP production within the PNSAs; all of these events were attenuated by MnTE-2-PyP(5+). Our results provide continued support for the role of mitotoxicity in the development of CIPN across chemotherapeutic drug classes, and identify peroxynitrite as a key mediator in these processes, thereby providing the rationale towards development of "peroxynitrite-targeted" therapeutics for CIPN.
Keywords: ATP depletion; Bortezomib; Chemotherapy-induced painful peripheral neuropathy; Mitochondrial dysfunction; Neuropathic pain; Oxaliplatin; Paclitaxel; Peripheral nerve sensory axons; Peroxynitrite; Superoxide dismutase.
Copyright © 2013 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures
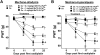
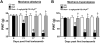

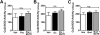

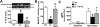
References
-
- Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso-tetrakis(ortho-N-alkylpyridyl)porphyrins. Synthesis, characterization, and catalysis of dismutation. Dalton Trans. 2002;13:2689–96.
-
- Bazzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. 2nd. Boston MA: Jones and Bartlett Publishers; 1999. pp. 459–90.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

