Active decorrelation in the basal ganglia
- PMID: 23892007
- PMCID: PMC3772785
- DOI: 10.1016/j.neuroscience.2013.07.032
Active decorrelation in the basal ganglia
Abstract
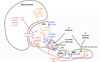
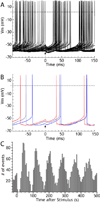
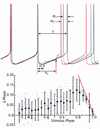
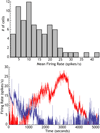
The cytoarchitecturally-homogeneous appearance of the globus pallidus, subthalamic nucleus and substantia nigra has long been said to imply a high degree of afferent convergence and sharing of inputs by nearby neurons. Moreover, axon collaterals of neurons in the external segment of the globus pallidus and the substantia nigra pars reticulata arborize locally and make inhibitory synapses on other cells of the same type. These features suggest that the connectivity of the basal ganglia may impose spike-time correlations among the cells, and it has been puzzling that experimental studies have failed to demonstrate such correlations. One possible solution arises from studies of firing patterns in basal ganglia cells, which reveal that they are nearly all pacemaker cells. Their high rate of firing does not depend on synaptic excitation, but they fire irregularly because a dense barrage of synaptic inputs normally perturbs the timing of their autonomous activity. Theoretical and computational studies show that the responses of repetitively-firing neurons to shared input or mutual synaptic coupling often defy classical intuitions about temporal synaptic integration. The patterns of spike-timing among such neurons depend on the ionic mechanism of pacemaking, the level of background uncorrelated cellular and synaptic noise, and the firing rates of the neurons, as well as the properties of their synaptic connections. Application of these concepts to the basal ganglia circuitry suggests that the connectivity and physiology of these nuclei may be configured to prevent the establishment of permanent spike-timing relationships between neurons. The development of highly synchronous oscillatory patterns of activity in Parkinson's disease may result from the loss of pacemaking by some basal ganglia neurons, and accompanying breakdown of the mechanisms responsible for active decorrelation.
Keywords: 6-OHDA; 6-hydroxy dopamine; EEG; GPe; GPi; HCN; Parkinson’s disease; SNr; STN; electroencephalographic; external segments of the globus pallidus; hyperpolarization-activated cation current; internal segments of the globus pallidus; network oscillations; phase-resetting; spike-timing; substantia nigra pars reticulata; subthalamic nucleus; synchrony.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Indirect pathway control of firing rate and pattern in the substantia nigra pars reticulata.J Neurophysiol. 2020 Feb 1;123(2):800-814. doi: 10.1152/jn.00678.2019. Epub 2020 Jan 15. J Neurophysiol. 2020. PMID: 31940230 Free PMC article.
-
Propagation of Oscillations in the Indirect Pathway of the Basal Ganglia.J Neurosci. 2023 Aug 30;43(35):6112-6125. doi: 10.1523/JNEUROSCI.0445-23.2023. Epub 2023 Jul 3. J Neurosci. 2023. PMID: 37400253 Free PMC article.
-
Substantia nigra control of basal ganglia nuclei.J Neural Transm Suppl. 2009;(73):91-101. doi: 10.1007/978-3-211-92660-4_7. J Neural Transm Suppl. 2009. PMID: 20411770 Review.
-
Basal ganglia control of substantia nigra dopaminergic neurons.J Neural Transm Suppl. 2009;(73):71-90. doi: 10.1007/978-3-211-92660-4_6. J Neural Transm Suppl. 2009. PMID: 20411769 Review.
-
Transmission of delta band (0.5-4 Hz) oscillations from the globus pallidus to the substantia nigra pars reticulata in dopamine depletion.J Comput Neurosci. 2022 Aug;51(3):361-380. doi: 10.1007/s10827-023-00853-z. Epub 2023 Jun 2. J Comput Neurosci. 2022. PMID: 37266768 Free PMC article.
Cited by
-
Discussion of Research Priorities for Gait Disorders in Parkinson's Disease.Mov Disord. 2022 Feb;37(2):253-263. doi: 10.1002/mds.28883. Epub 2021 Dec 22. Mov Disord. 2022. PMID: 34939221 Free PMC article. Review.
-
The highs and lows of beta activity in cortico-basal ganglia loops.Eur J Neurosci. 2014 Jun;39(11):1951-9. doi: 10.1111/ejn.12574. Epub 2014 Apr 3. Eur J Neurosci. 2014. PMID: 24890470 Free PMC article. Review.
-
Mechanisms of Network Interactions for Flexible Cortico-Basal Ganglia-Mediated Action Control.eNeuro. 2021 Jun 11;8(3):ENEURO.0009-21.2021. doi: 10.1523/ENEURO.0009-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33883192 Free PMC article.
-
Beta-frequency sensory stimulation enhances gait rhythmicity through strengthened coupling between striatal networks and stepping movement.Nat Commun. 2024 Sep 27;15(1):8336. doi: 10.1038/s41467-024-52664-0. Nat Commun. 2024. PMID: 39333151 Free PMC article.
-
Indirect pathway control of firing rate and pattern in the substantia nigra pars reticulata.J Neurophysiol. 2020 Feb 1;123(2):800-814. doi: 10.1152/jn.00678.2019. Epub 2020 Jan 15. J Neurophysiol. 2020. PMID: 31940230 Free PMC article.
References
-
- Abouzeid A, Ermentrout B. Type-II phase resetting curve is optimal for stochastic synchrony. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80:011911. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

