Measurement of acetylation turnover at distinct lysines in human histones identifies long-lived acetylation sites
- PMID: 23892279
- PMCID: PMC3929392
- DOI: 10.1038/ncomms3203
Measurement of acetylation turnover at distinct lysines in human histones identifies long-lived acetylation sites
Abstract
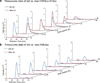
Histone acetylation has long been determined as a highly dynamic modification associated with open chromatin and transcriptional activation. Here we develop a metabolic labelling scheme using stable isotopes to study the kinetics of acetylation turnover at 19 distinct lysines on histones H3, H4 and H2A. Using human HeLa S3 cells, the analysis reveals 12 sites of histone acetylation with fast turnover and 7 sites stable over a 30 h experiment. The sites showing fast turnover (anticipated from classical radioactive measurements of whole histones) have half-lives between ~1-2 h. To support this finding, we use a broad-spectrum deacetylase inhibitor to verify that only fast turnover sites display 2-10-fold increases in acetylation whereas long-lived sites clearly do not. Most of these stable sites lack extensive functional studies or localization within global chromatin, and their role in non-genetic mechanisms of inheritance is as yet unknown.
Figures




References
-
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annual review of biochemistry. 2009;78:273–304. - PubMed
-
- Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annual review of biochemistry. 2011;80:473–499. - PubMed
-
- Isenberg I. Histones. Annual review of biochemistry. 1979;48:159–191. - PubMed
-
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annual review of biochemistry. 2007;76:75–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

