A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome
- PMID: 23892605
- PMCID: PMC3837522
- DOI: 10.1038/ng.2714
A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome
Abstract
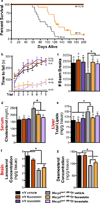
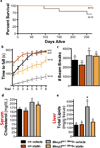
Mutations in MECP2, encoding methyl CpG-binding protein 2, cause Rett syndrome, the most severe autism spectrum disorder. Re-expressing Mecp2 in symptomatic Mecp2-null mice markedly improves function and longevity, providing hope that therapeutic intervention is possible in humans. To identify pathways in disease pathology for therapeutic intervention, we carried out a dominant N-ethyl-N-nitrosourea (ENU) mutagenesis suppressor screen in Mecp2-null mice and isolated five suppressors that ameliorate the symptoms of Mecp2 loss. We show that a stop codon mutation in Sqle, encoding squalene epoxidase, a rate-limiting enzyme in cholesterol biosynthesis, underlies suppression in one line. Subsequently, we also show that lipid metabolism is perturbed in the brains and livers of Mecp2-null male mice. Consistently, statin drugs improve systemic perturbations of lipid metabolism, alleviate motor symptoms and confer increased longevity in Mecp2 mutant mice. Our genetic screen therefore points to cholesterol homeostasis as a potential target for the treatment of patients with Rett syndrome.
Figures
Comment in
-
Cholesterol metabolism and Rett syndrome pathogenesis.Nat Genet. 2013 Sep;45(9):965-7. doi: 10.1038/ng.2738. Nat Genet. 2013. PMID: 23985682
-
Cholesterol metabolism is a potential therapeutic target for Rett syndrome.Clin Genet. 2014 Mar;85(3):229-30. doi: 10.1111/cge.12284. Epub 2013 Oct 10. Clin Genet. 2014. PMID: 24102619 No abstract available.
-
Neuroscience. Path to treat Rett syndrome.Science. 2013 Oct 18;342(6156):318-20. doi: 10.1126/science.1245657. Science. 2013. PMID: 24136956 No abstract available.
Similar articles
-
Suppressor mutations in Mecp2-null mice implicate the DNA damage response in Rett syndrome pathology.Genome Res. 2020 Apr;30(4):540-552. doi: 10.1101/gr.258400.119. Epub 2020 Apr 21. Genome Res. 2020. PMID: 32317254 Free PMC article.
-
MeCP2 co-ordinates liver lipid metabolism with the NCoR1/HDAC3 corepressor complex.Hum Mol Genet. 2016 Jul 15;25(14):3029-3041. doi: 10.1093/hmg/ddw156. Epub 2016 Jun 10. Hum Mol Genet. 2016. PMID: 27288453 Free PMC article.
-
Cholesterol metabolism and Rett syndrome pathogenesis.Nat Genet. 2013 Sep;45(9):965-7. doi: 10.1038/ng.2738. Nat Genet. 2013. PMID: 23985682
-
Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review.
-
Evolving role of MeCP2 in Rett syndrome and autism.Epigenomics. 2009 Oct;1(1):119-30. doi: 10.2217/epi.09.13. Epigenomics. 2009. PMID: 20473347 Free PMC article. Review.
Cited by
-
The Degron Architecture of Squalene Monooxygenase and How Specific Lipids Calibrate Levels of This Key Cholesterol Synthesis Enzyme.Adv Exp Med Biol. 2021;21:1-12. doi: 10.1007/5584_2020_583. Adv Exp Med Biol. 2021. PMID: 32979157
-
MeCP2 Related Studies Benefit from the Use of CD1 as Genetic Background.PLoS One. 2016 Apr 20;11(4):e0153473. doi: 10.1371/journal.pone.0153473. eCollection 2016. PLoS One. 2016. PMID: 27097329 Free PMC article.
-
Failure to identify modifiers of NEBULIN-related nemaline myopathy in two pre-clinical models of the disease.Biol Open. 2019 Sep 18;8(9):bio044867. doi: 10.1242/bio.044867. Biol Open. 2019. PMID: 31530540 Free PMC article.
-
Absence of strong strain effects in behavioral analyses of Shank3-deficient mice.Dis Model Mech. 2014 Jun;7(6):667-81. doi: 10.1242/dmm.013821. Epub 2014 Mar 20. Dis Model Mech. 2014. PMID: 24652766 Free PMC article.
-
Alterations in the carnitine cycle in a mouse model of Rett syndrome.Sci Rep. 2017 Feb 2;7:41824. doi: 10.1038/srep41824. Sci Rep. 2017. PMID: 28150739 Free PMC article.
References
-
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature genetics. 1999;23:185–188. - PubMed
-
- Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nature reviews. 2006;7:415–426. - PubMed
-
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature genetics. 2001;27:322–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

