Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease
- PMID: 23892607
- PMCID: PMC3758452
- DOI: 10.1038/ng.2715
Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease
Abstract
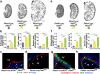
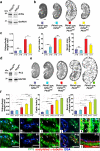
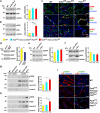
Kidney cysts occur following inactivation of polycystins in otherwise intact cilia or following complete removal of cilia by inactivation of intraflagellar transport-related proteins. We investigated the mechanisms of cyst formation in these two distinct processes by combining conditional inactivation of polycystins with concomitant ablation of cilia in developing and adult kidney and liver. We found that loss of intact cilia suppressed cyst growth following inactivation of polycystins and that the severity of cystic disease was directly related to the length of time between the initial loss of the polycystin proteins and the subsequent involution of cilia. This cilia-dependent cyst growth was not explained by activation of the MAPK/ERK, mTOR or cAMP pathways and is likely to be distinct from the mechanism of cyst growth following complete loss of cilia. These data establish the existence of a new pathway defined by polycystin-dependent inhibition and cilia-dependent activation that promotes rapid cyst growth.
Figures


Comment in
-
Polycystic kidney disease: Cyst growth and cilia in ADPKD.Nat Rev Nephrol. 2013 Oct;9(10):555. doi: 10.1038/nrneph.2013.160. Epub 2013 Aug 13. Nat Rev Nephrol. 2013. PMID: 23938593 No abstract available.
-
Novel cilia-dependent pathway activates rapid cyst growth in autosomal dominant polycystic kidney disease.Clin Genet. 2014 Mar;85(3):230-2. doi: 10.1111/cge.12285. Epub 2013 Oct 13. Clin Genet. 2014. PMID: 24111686 No abstract available.
References
-
- Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat.Rev.Nephrol. 2011 - PubMed
-
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. - PubMed
-
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

