An extracellular Serine/Threonine-rich protein from Lactobacillus plantarum NCIMB 8826 is a novel aggregation-promoting factor with affinity to mucin
- PMID: 23892754
- PMCID: PMC3811371
- DOI: 10.1128/AEM.01657-13
An extracellular Serine/Threonine-rich protein from Lactobacillus plantarum NCIMB 8826 is a novel aggregation-promoting factor with affinity to mucin
Abstract
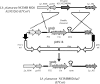
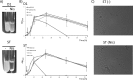
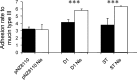
Autoaggregation in lactic acid bacteria is directly related to the production of certain extracellular proteins, notably, aggregation-promoting factors (APFs). Production of aggregation-promoting factors confers beneficial traits to probiotic-producing strains, contributing to their fitness for the intestinal environment. Furthermore, coaggregation with pathogens has been proposed to be a beneficial mechanism in probiotic lactic acid bacteria. This mechanism would limit attachment of the pathogen to the gut mucosa, favoring its removal by the human immune system. In the present paper, we have characterized a novel aggregation-promoting factor in Lactobacillus plantarum. A mutant with a knockout of the D1 gene showed loss of its autoaggregative phenotype and a decreased ability to bind to mucin, indicating an adhesion role of this protein. In addition, heterologous production of the D1 protein or an internal fragment of the protein, characterized by its abundance in serine/threonine, strongly induced autoaggregation in Lactococcus lactis. This result strongly suggested that this internal fragment is responsible for the bioactivity of D1 as an APF. To our knowledge, this is the first report on a gene coding for an aggregation-promoting factor in Lb. plantarum.
Figures

Similar articles
-
Lactobacillus plantarum extracellular chitin-binding protein and its role in the interaction between chitin, Caco-2 cells, and mucin.Appl Environ Microbiol. 2011 Feb;77(3):1123-6. doi: 10.1128/AEM.02080-10. Epub 2010 Dec 3. Appl Environ Microbiol. 2011. PMID: 21131525 Free PMC article.
-
The expression of adhesin EF-Tu in response to mucin and its role in Lactobacillus adhesion and competitive inhibition of enteropathogens to mucin.J Appl Microbiol. 2013 Aug;115(2):546-54. doi: 10.1111/jam.12249. Epub 2013 Jun 3. J Appl Microbiol. 2013. PMID: 23663754
-
Molecular Switch Controlling Expression of the Mannose-Specific Adhesin, Msa, in Lactobacillus plantarum.Appl Environ Microbiol. 2019 May 2;85(10):e02954-18. doi: 10.1128/AEM.02954-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30877113 Free PMC article.
-
Lactobacillus plantarum and Its Probiotic and Food Potentialities.Probiotics Antimicrob Proteins. 2017 Jun;9(2):111-122. doi: 10.1007/s12602-017-9264-z. Probiotics Antimicrob Proteins. 2017. PMID: 28271469 Review.
-
How do they stick together? Bacterial adhesins implicated in the binding of bacteria to the human gastrointestinal mucins.Biochem Soc Trans. 2017 Apr 15;45(2):389-399. doi: 10.1042/BST20160167. Biochem Soc Trans. 2017. PMID: 28408479 Review.
Cited by
-
Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks.Animals (Basel). 2023 Sep 19;13(18):2958. doi: 10.3390/ani13182958. Animals (Basel). 2023. PMID: 37760358 Free PMC article.
-
Butanol Tolerance of Lactiplantibacillus plantarum: A Transcriptome Study.Genes (Basel). 2021 Jan 27;12(2):181. doi: 10.3390/genes12020181. Genes (Basel). 2021. PMID: 33514005 Free PMC article.
-
Bacterial autoaggregation.AIMS Microbiol. 2018 Mar 1;4(1):140-164. doi: 10.3934/microbiol.2018.1.140. eCollection 2018. AIMS Microbiol. 2018. PMID: 31294207 Free PMC article. Review.
-
Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni.PLoS One. 2014 Sep 29;9(9):e108827. doi: 10.1371/journal.pone.0108827. eCollection 2014. PLoS One. 2014. PMID: 25264604 Free PMC article.
-
Lactobacillus plantarum WCFS1 and its host interaction: a dozen years after the genome.Microb Biotechnol. 2016 Jul;9(4):452-65. doi: 10.1111/1751-7915.12368. Epub 2016 May 27. Microb Biotechnol. 2016. PMID: 27231133 Free PMC article. Review.
References
-
- Chevallier B, Hubert JC, Kammerer B. 1994. Determination of chromosome size and number of rrn loci in Lactobacillus plantarum by pulsed-field gel-electrophoresis. FEMS Microbiol. Lett. 120:51–56 - PubMed
-
- Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RMK, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. U. S. A. 100:1990–1995 - PMC - PubMed
-
- Tallon R, Arias S, Bressollier P, Urdaci MC. 2007. Strain- and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J. Appl. Microbiol. 102:442–451 - PubMed
-
- de Vries MC, Vaughan EE, Kleerebezem M, de Vos WM. 2006. Lactobacillus plantarum—survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 16:1018–1028
-
- Molin G. 2001. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73:380S–385S - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

