Recovery from slow inactivation in K+ channels is controlled by water molecules
- PMID: 23892782
- PMCID: PMC3799803
- DOI: 10.1038/nature12395
Recovery from slow inactivation in K+ channels is controlled by water molecules
Abstract
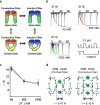
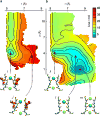
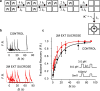
Application of a specific stimulus opens the intracellular gate of a K(+) channel (activation), yielding a transient period of ion conduction until the selectivity filter spontaneously undergoes a conformational change towards a non-conductive state (inactivation). Removal of the stimulus closes the gate and allows the selectivity filter to interconvert back to its conductive conformation (recovery). Given that the structural differences between the conductive and inactivated filter are very small, it is unclear why the recovery process can take up to several seconds. The bacterial K(+) channel KcsA from Streptomyces lividans can be used to help elucidate questions about channel inactivation and recovery at the atomic level. Although KcsA contains only a pore domain, without voltage-sensing machinery, it has the structural elements necessary for ion conduction, activation and inactivation. Here we reveal, by means of a series of long molecular dynamics simulations, how the selectivity filter is sterically locked in the inactive conformation by buried water molecules bound behind the selectivity filter. Potential of mean force calculations show how the recovery process is affected by the buried water molecules and the rebinding of an external K(+) ion. A kinetic model deduced from the simulations shows how releasing the buried water molecules can stretch the timescale of recovery to seconds. This leads to the prediction that reducing the occupancy of the buried water molecules by imposing a high osmotic stress should accelerate the rate of recovery, which was verified experimentally by measuring the recovery rate in the presence of a 2-molar sucrose concentration.
Figures

References
-
- Cordero-Morales JF, Cuello LG, Zhao YX, Jogini V, Cortes DM, Roux B, Perozo E. Molecular determinants of gating at the potassium-channel selectivity filter. Nature Structural & Molecular Biology. 2006;13:311–318. - PubMed
-
- Cordero-Morales JF, Jogini V, Lewis A, Vasquez V, Cortes DM, Roux B, Perozo E. Molecular driving forces determining potassium channel slow inactivation. Nature Structure and Molecular Biology. 2007;14:1062–1069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

