Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context
- PMID: 23893167
- PMCID: PMC3798751
- DOI: 10.1152/ajpheart.00434.2013
Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context
Abstract
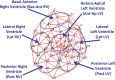
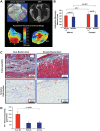
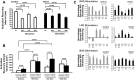
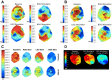
Myocardial infarction (MI) induces neural and electrical remodeling at scar border zones. The impact of focal MI on global functional neural remodeling is not well understood. Sympathetic stimulation was performed in swine with anteroapical infarcts (MI; n = 9) and control swine (n = 9). A 56-electrode sock was placed over both ventricles to record electrograms at baseline and during left, right, and bilateral stellate ganglion stimulation. Activation recovery intervals (ARIs) were measured from electrograms. Global and regional ARI shortening, dispersion of repolarization, and activation propagation were assessed before and during sympathetic stimulation. At baseline, mean ARI was shorter in MI hearts than control hearts (365 ± 8 vs. 436 ± 9 ms, P < 0.0001), dispersion of repolarization was greater in MI versus control hearts (734 ± 123 vs. 362 ± 32 ms(2), P = 0.02), and the infarcted region in MI hearts showed longer ARIs than noninfarcted regions (406 ± 14 vs. 365 ± 8 ms, P = 0.027). In control animals, percent ARI shortening was greater on anterior than posterior walls during right stellate ganglion stimulation (P = 0.0001), whereas left stellate ganglion stimulation showed the reverse (P = 0.0003). In infarcted animals, this pattern was completely lost. In 50% of the animals studied, sympathetic stimulation, compared with baseline, significantly altered the direction of activation propagation emanating from the intramyocardial scar during pacing. In conclusion, focal distal anterior MI alters regional and global pattern of sympathetic innervation, resulting in shorter ARIs in infarcted hearts, greater repolarization dispersion, and altered activation propagation. These conditions may underlie the mechanisms by which arrhythmias are initiated when sympathetic tone is enhanced.
Keywords: autonomic nervous system; cardiac innervation; neural remodeling; sympathetic nerves.
Figures

References
-
- Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm 7: 994–996, 2010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

