A novel p38 mitogen-activated protein kinase/Elk-1 transcription factor-dependent molecular mechanism underlying abnormal endothelial cell proliferation in plexogenic pulmonary arterial hypertension
- PMID: 23893408
- PMCID: PMC3764778
- DOI: 10.1074/jbc.M113.502674
A novel p38 mitogen-activated protein kinase/Elk-1 transcription factor-dependent molecular mechanism underlying abnormal endothelial cell proliferation in plexogenic pulmonary arterial hypertension
Erratum in
- J Biol Chem. 2013 Dec 27;288(52):36855. Ryan-Hemnes, Anna [corrected to Hemnes, Anna Ryan]
Abstract
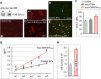
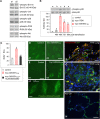
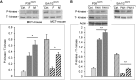
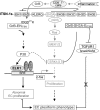
Plexiform lesions (PLs), the hallmark of plexogenic pulmonary arterial hypertension (PAH), contain phenotypically altered, proliferative endothelial cells (ECs). The molecular mechanism that contributes to EC proliferation and formation of PLs is poorly understood. We now show that a decrease in intersectin-1s (ITSN-1s) expression due to granzyme B (GrB) cleavage during inflammation associated with PAH and the high p38/Erk1/2(MAPK) activity ratio caused by the GrB/ITSN cleavage products lead to EC proliferation and selection of a proliferative/plexiform EC phenotype. We used human pulmonary artery ECs of PAH subjects (EC(PAH)), paraffin-embedded and frozen human lung tissue, and animal models of PAH in conjunction with microscopy imaging, biochemical, and molecular biology approaches to demonstrate that GrB cleaves ITSN-1s, a prosurvival protein of lung ECs, and generates two biologically active fragments, an N-terminal fragment (GrB-EH(ITSN)) with EC proliferative potential and a C-terminal product with dominant negative effects on Ras/Erk1/2. The proliferative potential of GrB-EH(ITSN) is mediated via sustained phosphorylation of p38(MAPK) and Elk-1 transcription factor and abolished by chemical inhibition of p38(MAPK). Moreover, lung tissue of PAH animal models and human specimens and EC(PAH) express lower levels of ITSN-1s compared with controls and the GrB-EH(ITSN) cleavage product. Moreover, GrB immunoreactivity is associated with PLs in PAH lungs. The concurrent expression of the two cleavage products results in a high p38/Erk1/2(MAPK) activity ratio, which is critical for EC proliferation. Our findings identify a novel GrB-EH(ITSN)-dependent pathogenic p38(MAPK)/Elk-1 signaling pathway involved in the poorly understood process of PL formation in severe PAH.
Keywords: Endothelium; Proliferation; Pulmonary Hypertension; Vascular Biology; p38 MAPK.
Figures





References
-
- Voelkel N. F., Quaife R. A., Leinwand L. A., Barst R. J., McGoon M. D., Meldrum D. R., Dupuis J., Long C. S., Rubin L. J., Smart F. W., Suzuki Y. J., Gladwin M., Denholm E. M., Gail D. B. (2006) Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114, 1883–1891 - PubMed
-
- Masri F. A., Xu W., Comhair S. A., Asosingh K., Koo M., Vasanji A., Drazba J., Anand-Apte B., Erzurum S. C. (2007) Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L548–L554 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

