Escherichia coli F1Fo-ATP synthase with a b/δ fusion protein allows analysis of the function of the individual b subunits
- PMID: 23893411
- PMCID: PMC3772190
- DOI: 10.1074/jbc.M113.503722
Escherichia coli F1Fo-ATP synthase with a b/δ fusion protein allows analysis of the function of the individual b subunits
Abstract
The "stator stalk" of F1Fo-ATP synthase is essential for rotational catalysis as it connects the nonrotating portions of the enzyme. In Escherichia coli, the stator stalk consists of two (identical) b subunits and the δ subunit. In mycobacteria, one of the b subunits and the δ subunit are replaced by a b/δ fusion protein; the remaining b subunit is of the shorter b' type. In the present study, it is shown that it is possible to generate a functional E. coli ATP synthase containing a b/δ fusion protein. This construct allowed the analysis of the roles of the individual b subunits. The full-length b subunit (which in this case is covalently linked to δ in the fusion protein) is responsible for connecting the stalk to the catalytic F1 subcomplex. It is not required for interaction with the membrane-embedded Fo subcomplex, as its transmembrane helix can be removed. Attachment to Fo is the function of the other b subunit which in turn has only a minor (if any at all) role in binding to δ. Also in E. coli the second b subunit can be shortened to a b' type.
Keywords: ATP Synthase; Enzyme Catalysis; Enzyme Mechanisms; Membrane Proteins; Protein Assembly.
Figures

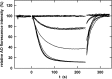
Similar articles
-
Assembly of the stator in Escherichia coli ATP synthase. Complexation of alpha subunit with other F1 subunits is prerequisite for delta subunit binding to the N-terminal region of alpha.Biochemistry. 2006 Dec 26;45(51):15893-902. doi: 10.1021/bi0619730. Epub 2006 Dec 5. Biochemistry. 2006. PMID: 17176112 Free PMC article.
-
Individual interactions of the b subunits within the stator of the Escherichia coli ATP synthase.J Biol Chem. 2013 Aug 23;288(34):24465-79. doi: 10.1074/jbc.M113.465633. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846684 Free PMC article.
-
Interaction with monomeric subunit c drives insertion of ATP synthase subunit a into the membrane and primes a-c complex formation.J Biol Chem. 2011 Nov 4;286(44):38583-38591. doi: 10.1074/jbc.M111.294868. Epub 2011 Sep 7. J Biol Chem. 2011. PMID: 21900248 Free PMC article.
-
ATP synthase: subunit-subunit interactions in the stator stalk.Biochim Biophys Acta. 2006 Sep-Oct;1757(9-10):1162-70. doi: 10.1016/j.bbabio.2006.04.007. Epub 2006 Apr 19. Biochim Biophys Acta. 2006. PMID: 16730323 Free PMC article. Review.
-
ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols.Biochim Biophys Acta. 2016 Feb;1857(2):129-140. doi: 10.1016/j.bbabio.2015.11.005. Epub 2015 Nov 14. Biochim Biophys Acta. 2016. PMID: 26589785 Review.
Cited by
-
Mycobacterial Membrane Proteins QcrB and AtpE: Roles in Energetics, Antibiotic Targets, and Associated Mechanisms of Resistance.J Membr Biol. 2018 Feb;251(1):105-117. doi: 10.1007/s00232-017-9997-3. Epub 2017 Nov 2. J Membr Biol. 2018. PMID: 29098330
-
Interaction between γC87 and γR242 residues participates in energy coupling between catalysis and proton translocation in Escherichia coli ATP synthase.Biochim Biophys Acta Bioenerg. 2019 Aug 1;1860(8):679-687. doi: 10.1016/j.bbabio.2019.06.016. Epub 2019 Jun 25. Biochim Biophys Acta Bioenerg. 2019. PMID: 31251901 Free PMC article.
-
Mycobacterium tuberculosis F-ATP Synthase Inhibitors and Targets.Antibiotics (Basel). 2024 Dec 3;13(12):1169. doi: 10.3390/antibiotics13121169. Antibiotics (Basel). 2024. PMID: 39766559 Free PMC article. Review.
-
Structural Elements Involved in ATP Hydrolysis Inhibition and ATP Synthesis of Tuberculosis and Nontuberculous Mycobacterial F-ATP Synthase Decipher New Targets for Inhibitors.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0105622. doi: 10.1128/aac.01056-22. Epub 2022 Nov 29. Antimicrob Agents Chemother. 2022. PMID: 36445139 Free PMC article.
-
Evolution of the F0F1 ATP synthase complex in light of the patchy distribution of different bioenergetic pathways across prokaryotes.PLoS Comput Biol. 2014 Sep 4;10(9):e1003821. doi: 10.1371/journal.pcbi.1003821. eCollection 2014 Sep. PLoS Comput Biol. 2014. PMID: 25188293 Free PMC article.
References
-
- Weber J., Senior A. E. (2003) ATP synthesis driven by proton transport in F1Fo-ATP synthase. FEBS Lett. 545, 61–70 - PubMed
-
- Junge W., Sielaff H., Engelbrecht S. (2009) Torque generation and elastic power transmission in the rotary FoF1-ATPase. Nature 459, 364–370 - PubMed
-
- Nakanishi-Matsui M., Sekiya M., Nakamoto R. K., Futai M. (2010) The mechanism of rotating proton pumping ATPases. Biochim. Biophys. Acta 1797, 1343–1352 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

