β-Hairpin loop of eukaryotic initiation factor 1 (eIF1) mediates 40 S ribosome binding to regulate initiator tRNA(Met) recruitment and accuracy of AUG selection in vivo
- PMID: 23893413
- PMCID: PMC3779751
- DOI: 10.1074/jbc.M113.498642
β-Hairpin loop of eukaryotic initiation factor 1 (eIF1) mediates 40 S ribosome binding to regulate initiator tRNA(Met) recruitment and accuracy of AUG selection in vivo
Abstract
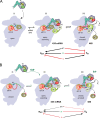
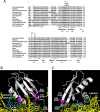
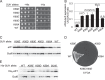
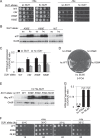
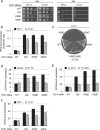
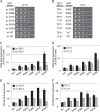
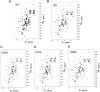
Recognition of the translation initiation codon is thought to require dissociation of eIF1 from the 40 S ribosomal subunit, enabling irreversible GTP hydrolysis (Pi release) by the eIF2·GTP·Met-tRNAi ternary complex (TC), rearrangement of the 40 S subunit to a closed conformation incompatible with scanning, and stable binding of Met-tRNAi to the P site. The crystal structure of a Tetrahymena 40 S·eIF1 complex revealed several basic amino acids in eIF1 contacting 18 S rRNA, and we tested the prediction that their counterparts in yeast eIF1 are required to prevent premature eIF1 dissociation from scanning ribosomes at non-AUG triplets. Supporting this idea, substituting Lys-60 in helix α1, or either Lys-37 or Arg-33 in β-hairpin loop-1, impairs binding of yeast eIF1 to 40 S·eIF1A complexes in vitro, and it confers increased initiation at UUG codons (Sui(-) phenotype) or lethality, in a manner suppressed by overexpressing the mutant proteins or by an eIF1A mutation (17-21) known to impede eIF1 dissociation in vitro. The eIF1 Sui(-) mutations also derepress translation of GCN4 mRNA, indicating impaired ternary complex loading, and this Gcd(-) phenotype is likewise suppressed by eIF1 overexpression or the 17-21 mutation. These findings indicate that direct contacts of eIF1 with 18 S rRNA seen in the Tetrahymena 40 S·eIF1 complex are crucial in yeast to stabilize the open conformation of the 40 S subunit and are required for rapid TC loading and ribosomal scanning and to impede rearrangement to the closed complex at non-AUG codons. Finally, we implicate the unstructured N-terminal tail of eIF1 in blocking rearrangement to the closed conformation in the scanning preinitiation complex.
Keywords: AUG Recognition; Ribosome Function; Ribosomes; Transfer RNA (tRNA); Translation; Translation Initiation Factors; eIF1; eIF2.
Figures



Similar articles
-
eIF1 Loop 2 interactions with Met-tRNAi control the accuracy of start codon selection by the scanning preinitiation complex.Proc Natl Acad Sci U S A. 2018 May 1;115(18):E4159-E4168. doi: 10.1073/pnas.1800938115. Epub 2018 Apr 16. Proc Natl Acad Sci U S A. 2018. PMID: 29666249 Free PMC article.
-
Enhanced eIF1 binding to the 40S ribosome impedes conformational rearrangements of the preinitiation complex and elevates initiation accuracy.RNA. 2014 Feb;20(2):150-67. doi: 10.1261/rna.042069.113. Epub 2013 Dec 13. RNA. 2014. PMID: 24335188 Free PMC article.
-
A network of eIF2β interactions with eIF1 and Met-tRNAi promotes accurate start codon selection by the translation preinitiation complex.Nucleic Acids Res. 2019 Mar 18;47(5):2574-2593. doi: 10.1093/nar/gky1274. Nucleic Acids Res. 2019. PMID: 30576497 Free PMC article.
-
The scanning mechanism of eukaryotic translation initiation.Annu Rev Biochem. 2014;83:779-812. doi: 10.1146/annurev-biochem-060713-035802. Epub 2014 Jan 29. Annu Rev Biochem. 2014. PMID: 24499181 Review.
-
Structural Insights into the Mechanism of Scanning and Start Codon Recognition in Eukaryotic Translation Initiation.Trends Biochem Sci. 2017 Aug;42(8):589-611. doi: 10.1016/j.tibs.2017.03.004. Epub 2017 Apr 22. Trends Biochem Sci. 2017. PMID: 28442192 Review.
Cited by
-
Inhibitors of eIF4G1-eIF1 uncover its regulatory role of ER/UPR stress-response genes independent of eIF2α-phosphorylation.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2120339119. doi: 10.1073/pnas.2120339119. Epub 2022 Jul 20. Proc Natl Acad Sci U S A. 2022. PMID: 35857873 Free PMC article.
-
Role of aIF1 in Pyrococcus abyssi translation initiation.Nucleic Acids Res. 2018 Nov 16;46(20):11061-11074. doi: 10.1093/nar/gky850. Nucleic Acids Res. 2018. PMID: 30239976 Free PMC article.
-
Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core.Int J Mol Sci. 2019 Feb 21;20(4):939. doi: 10.3390/ijms20040939. Int J Mol Sci. 2019. PMID: 30795538 Free PMC article. Review.
-
eIF1A residues implicated in cancer stabilize translation preinitiation complexes and favor suboptimal initiation sites in yeast.Elife. 2017 Dec 5;6:e31250. doi: 10.7554/eLife.31250. Elife. 2017. PMID: 29206102 Free PMC article.
-
eIF2α interactions with mRNA control accurate start codon selection by the translation preinitiation complex.Nucleic Acids Res. 2020 Oct 9;48(18):10280-10296. doi: 10.1093/nar/gkaa761. Nucleic Acids Res. 2020. PMID: 32955564 Free PMC article.
References
-
- Pestova T. V., Lorsch J. R., Hellen C. U. T. (2007) in Translational Control in Biology and Medicine (Mathews M., Sonenberg N., Hershey J. W., eds) pp. 87–128, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Algire M. A., Maag D., Lorsch J. R. (2005) Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20, 251–262 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

