Covalent trapping of methyllycaconitine at the α4-α4 interface of the α4β2 nicotinic acetylcholine receptor: antagonist binding site and mode of receptor inhibition revealed
- PMID: 23893416
- PMCID: PMC3772200
- DOI: 10.1074/jbc.M113.475053
Covalent trapping of methyllycaconitine at the α4-α4 interface of the α4β2 nicotinic acetylcholine receptor: antagonist binding site and mode of receptor inhibition revealed
Abstract
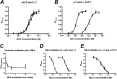
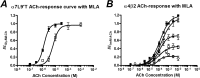
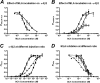
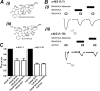
The α4β2 nicotinic acetylcholine receptors (nAChRs) are widely expressed in the brain and are implicated in a variety of physiological processes. There are two stoichiometries of the α4β2 nAChR, (α4)2(β2)3 and (α4)3(β2)2, with different sensitivities to acetylcholine (ACh), but their pharmacological profiles are not fully understood. Methyllycaconitine (MLA) is known to be an antagonist of nAChRs. Using the two-electrode voltage clamp technique and α4β2 nAChRs in the Xenopus oocyte expression system, we demonstrate that inhibition by MLA occurs via two different mechanisms; that is, a direct competitive antagonism and an apparently insurmountable mechanism that only occurs after preincubation with MLA. We hypothesized an additional MLA binding site in the α4-α4 interface that is unique to this stoichiometry. To prove this, we covalently trapped a cysteine-reactive MLA analog at an α4β2 receptor containing an α4(D204C) mutation predicted by homology modeling to be within reach of the reactive probe. We demonstrate that covalent trapping results in irreversible reduction of ACh-elicited currents in the (α4)3(β2)2 stoichiometry, indicating that MLA binds to the α4-α4 interface of the (α4)3(β2)2 and providing direct evidence of ligand binding to the α4-α4 interface. Consistent with other studies, we propose that the α4-α4 interface is a structural target for potential therapeutics that modulate (α4)3(β2)2 nAChRs.
Keywords: Cys-loop Receptors; Cysteine Trapping; Homology Modeling; Molecular Pharmacology; Nicotinic Acetylcholine Receptors; Receptor Inhibition; Receptor Structure-Function.
Figures


Similar articles
-
Identifying the binding site of novel methyllycaconitine (MLA) analogs at α4β2 nicotinic acetylcholine receptors.ACS Chem Neurosci. 2010 Dec 15;1(12):796-809. doi: 10.1021/cn100073x. Epub 2010 Oct 7. ACS Chem Neurosci. 2010. PMID: 22778816 Free PMC article.
-
AE Succinimide, an Analogue of Methyllycaconitine, When Bound Generates a Nonconducting Conformation of the α4β2 Nicotinic Acetylcholine Receptor.ACS Chem Neurosci. 2020 Feb 5;11(3):344-355. doi: 10.1021/acschemneuro.9b00525. Epub 2020 Jan 14. ACS Chem Neurosci. 2020. PMID: 31898891
-
Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity.J Biol Chem. 2011 Sep 2;286(35):31043-31054. doi: 10.1074/jbc.M111.262014. Epub 2011 Jul 14. J Biol Chem. 2011. PMID: 21757735 Free PMC article.
-
The unique α4+/-α4 agonist binding site in (α4)3(β2)2 subtype nicotinic acetylcholine receptors permits differential agonist desensitization pharmacology versus the (α4)2(β2)3 subtype.J Pharmacol Exp Ther. 2014 Jan;348(1):46-58. doi: 10.1124/jpet.113.208389. Epub 2013 Nov 4. J Pharmacol Exp Ther. 2014. PMID: 24190916 Free PMC article.
-
Novel methyllycaconitine analogues selective for the α4β2 over α7 nicotinic acetylcholine receptors.Bioorg Med Chem. 2021 Dec 1;51:116516. doi: 10.1016/j.bmc.2021.116516. Epub 2021 Nov 10. Bioorg Med Chem. 2021. PMID: 34798380
Cited by
-
The fifth subunit of the (α4β2)2 β2 nicotinic ACh receptor modulates maximal ACh responses.Br J Pharmacol. 2018 Jun;175(11):1822-1837. doi: 10.1111/bph.13905. Epub 2017 Jul 14. Br J Pharmacol. 2018. PMID: 28600847 Free PMC article.
-
High-Throughput Patch Clamp Screening in Human α6-Containing Nicotinic Acetylcholine Receptors.SLAS Discov. 2017 Jul;22(6):686-695. doi: 10.1177/2472555217696794. Epub 2017 Mar 15. SLAS Discov. 2017. PMID: 28298165 Free PMC article.
-
Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors.Br J Pharmacol. 2018 Jun;175(11):1805-1821. doi: 10.1111/bph.13745. Epub 2017 Mar 20. Br J Pharmacol. 2018. PMID: 28199738 Free PMC article. Review.
-
Tying up Nicotine: New Selective Competitive Antagonist of the Neuronal Nicotinic Acetylcholine Receptors.ACS Med Chem Lett. 2015 Mar 4;6(4):472-5. doi: 10.1021/acsmedchemlett.5b00028. eCollection 2015 Apr 9. ACS Med Chem Lett. 2015. PMID: 25941557 Free PMC article.
-
Ligand Binding at the 4-4 Agonist-Binding Site of the 42 nAChR Triggers Receptor Activation through a Pre-Activated Conformational State.PLoS One. 2016 Aug 23;11(8):e0161154. doi: 10.1371/journal.pone.0161154. eCollection 2016. PLoS One. 2016. PMID: 27552221 Free PMC article.
References
-
- Gotti C., Clementi F. (2004) Neuronal nicotinic receptors. From structure to pathology. Prog. Neurobiol. 74, 363–396 - PubMed
-
- Karlin A. (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3, 102–114 - PubMed
-
- Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R, Wang H., Abumrad N., Eaton J. W., Tracey K. J. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 - PubMed
-
- Jensen A. A., Frølund B., Liljefors T., Krogsgaard-Larsen P. (2005) Neuronal nicotinic acetylcholine receptors. Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48, 4705–4745 - PubMed
-
- Grosman C., Zhou M., Auerbach A. (2000) Mapping the conformational wave of acetylcholine receptor channel gating. Nature 403, 773–776 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

